Label: MYCOZYL AL- tolnaftate liquid
- NDC Code(s): 59088-443-01
- Packager: PURETEK CORPORATION
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
Each gram of Mycozyl AL™ contains 10 mg of tolnaftate in a vehicle consisting of: Apple Cider Vinegar, Argania
Spinosa (Argan) Kernel Oil, Benzyl Alcohol, DMSO (Dimethyl Sulfoxide), Eucalyptus Globulus (Eucalyptus) Leaf
Oil, Glycerin, Laureth-4, Lavandula Angustifolia (Lavender) Oil, Melaleuca Alternifolia (Tea Tree) Leaf Oil, PEG-8,
DL-alpha-tocopheryl Acetate.
Chemically, tolnaftate molecular formula is C19H17NOS and molecular weight 314.5 and is represented by the
following structure formula: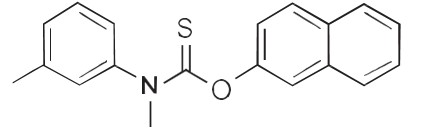
- CLINICAL PHARMACOLOGY:
- Pharmacokinetics:
-
INDICATIONS AND USAGE:
Mycozyl AL™ is effective in the treatment of most skin infections such as athlete's foot (tinea pedis) and ringworm (tinea corporis). Mycozyl AL™ has been designed to reach skin areas around and under the nails while it relives burning, cracking, scaling and discomfort which accompany these conditions. Mycozyl AL™ is an antifungal that works by preventing and eliminating the growth of fungus on fingers, toes and around the nails. It eliminates and helps stop the spread of fungal infections on cuticles around nail edges and under the nail tips where reachable with applicator brush. Mycozyl AL™ cures and prevents fungal infections from coming back with daily use.
- CONTRAINDICATIONS:
- PRECAUTIONS:
- WARNINGS:
- General:
- Carcinogenesis, Mutagenesis, and Impairment of Fertility:
- Pregnancy:
- Nursing Mothers:
- Pediatric Use:
- ADVERSE REACTIONS:
- OVERDOSAGE:
-
DOSAGE AND ADMINISTRATION:
• Clean the affected area with soap and warm water and dry thoroughly
• Apply a thin layer of product over affected area twice daily (morning and night) paying special attention to the edges of the nail, cuticles and skin around the nails or as directed by a doctor
• The brush applicator allows for easy application on skin around the nail and cuticle areas.
• For athlete's foot, pay special attention to spaces between the toes, wear well-fitting,ventilated shoes, and change shoes and socks at least once daily.
• For athlete's foot and ringworm, use daily for 4 weeks.
• Supervise children in the use of this product.
Use under the direction of a licensed medical practitioner.
Call your doctor about side effects. To report side effects, call PureTek Corporation at
1-877-921-7873 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. -
HOW SUPPLIED:
Mycozyl AL™ is supplied in:
10 mL glass bottle with a screw cap fitted with a brush applicator (NDC 59088-443-01).Store at 20º-25ºC (68º-77ºF) [see USP Controlled Room Temperature]. Protect from freezing and excessive heat. Keep
container tightly closed.
Do not use if package is damaged. Keep out of reach of children.
Manufactured by:
PureTek Corporation
Panorama City, CA 91402
For questions or information
call toll-free: 877-921-7873 - Mycozyl AL™
-
INGREDIENTS AND APPEARANCE
MYCOZYL AL
tolnaftate liquidProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59088-443 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 0.1 g in 10 mL Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TEA TREE OIL (UNII: VIF565UC2G) GLYCERIN (UNII: PDC6A3C0OX) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) BENZYL ALCOHOL (UNII: LKG8494WBH) LAURETH-4 (UNII: 6HQ855798J) APPLE CIDER VINEGAR (UNII: 0UE22Q87VC) EUCALYPTUS OIL (UNII: 2R04ONI662) ARGAN OIL (UNII: 4V59G5UW9X) LAVENDER OIL (UNII: ZBP1YXW0H8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59088-443-01 10 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/17/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/17/2023 Labeler - PURETEK CORPORATION (785961046)


