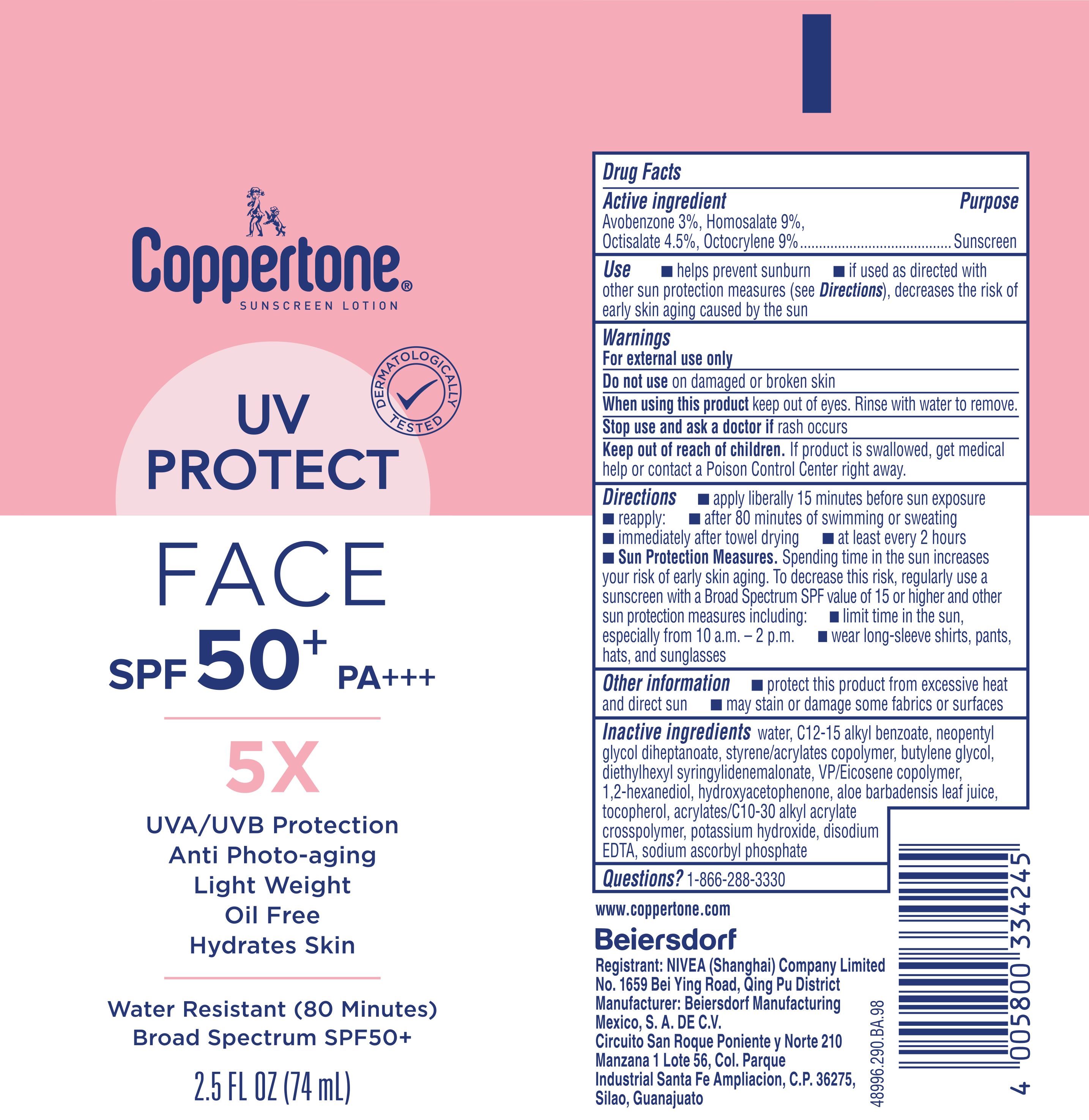
Label: COPPERTONE 5X UV PROTECT FACE SUNSCREEN- avobenzone 3%, homosalate 9%, octisalate 4.5%, octocrylene 9% lotion
- NDC Code(s): 66800-0067-2
- Packager: Beiersdorf Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated January 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purpose
- Uses
- Warnings
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
■ apply liberally 15 minutes before sun exposure
■ reapply:
■ after 80 minutes of swimming or sweating
■ immediately after towel drying
■ at least every 2 hours
■ Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
■ limit time in the sun, especially from 10 a.m. – 2 p.m.
■ wear long-sleeve shirts, pants, hats, and sunglasses
■ children under 6 months: Ask a doctor
- Other information
-
Inactive ingredients
water, C12-15 alkyl benzoate, neopentyl glycol diheptanoate, styrene/acrylates copolymer, butylene glycol, diethylhexyl syringylidenemalonate, VP/Eicosene copolymer, 1,2-hexanediol, hydroxyacetophenone, aloe barbadensis leaf juice, tocopherol, acrylates/C10-30 alkyl acrylate crosspolymer, potassium hydroxide, disodium EDTA, sodium ascorbyl phosphate
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COPPERTONE 5X UV PROTECT FACE SUNSCREEN
avobenzone 3%, homosalate 9%, octisalate 4.5%, octocrylene 9% lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66800-0067 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.5 g in 100 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 9 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 9 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 g Inactive Ingredients Ingredient Name Strength NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) TOCOPHEROL (UNII: R0ZB2556P8) DISODIUM EDTA-COPPER (UNII: 6V475AX06U) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) ALOE VERA LEAF (UNII: ZY81Z83H0X) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) WATER (UNII: 059QF0KO0R) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) DOCOSANOL (UNII: 9G1OE216XY) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) Product Characteristics Color white (White to Off-White) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66800-0067-2 74 g in 1 TUBE; Type 0: Not a Combination Product 11/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 11/01/2023 Labeler - Beiersdorf Inc (001177906)