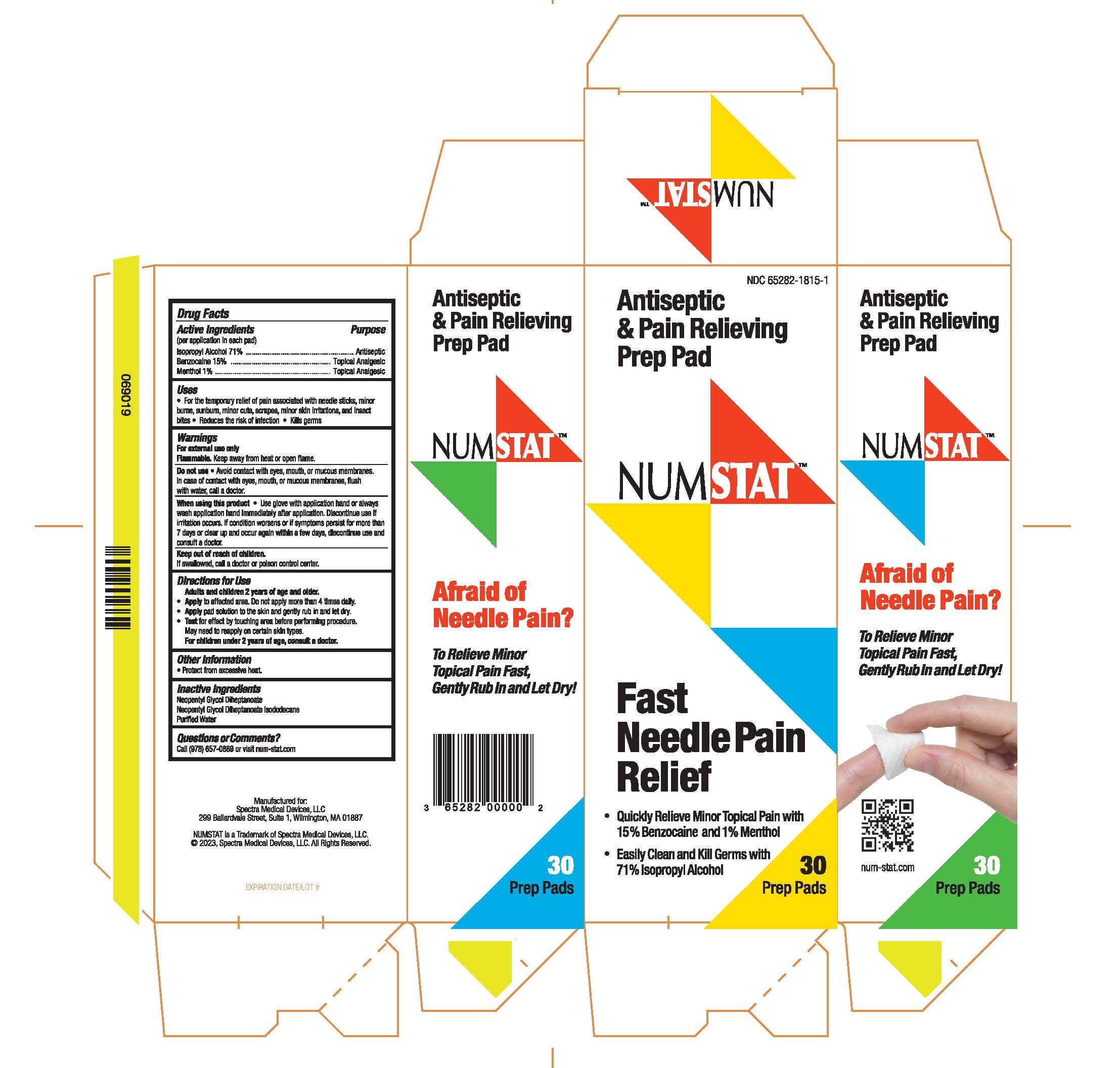
Label: NUMSTAT ANTISEPTIC AND PAIN RELIEVING PREP PAD- alcohol, menthol, and benzocaine prep pad swab
- NDC Code(s): 65282-1815-1, 65282-1815-2
- Packager: Spectra Medical Devices, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- Do Not Use
- Stop use
- Keep out of reach of children
-
Directions
Directions for Use
Adults and children 2 years of age and older.
• Apply to affected area. Do not apply more than 4 times daily.
• Apply pad solution to the skin and gently rub in and let dry.
• Test for effect by touching area before performing procedure
May need to reapply on certain skin types.
For children under 2 years of age, consult a doctor.
- Other Information
- Inactive Ingredients
- Questions or Comments?
-
Carton Information
Antiseptic & Pain Relieving Prep Pad
NUMSTAT
Fast Needle Pain Relief
• Quickly Relieve Minor Topical Pain with 15% Benzocaine and 1% Menthol
• Easily Clean and Kill Germs with 71% Isopropyl Alcohol
Afraid of Needle Pain?
To Relieve Minor
Topical Pain Fast,
Gently Rub In and Let Dry!
Manufactured for:
Spectra Medical Devices, LLC
299 Ballardvale Street, Suite 1, Wilmington, MA 01887
NUMSTAT (TM) and © Copyright 2023
Spectra Medical Devices LLC, All Rights Reserved.
- Package Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
NUMSTAT ANTISEPTIC AND PAIN RELIEVING PREP PAD
alcohol, menthol, and benzocaine prep pad swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65282-1815 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 15 g in 100 g ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 71 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65282-1815-1 30 in 1 CARTON 01/01/2023 1 1 g in 1 PACKET; Type 0: Not a Combination Product 2 NDC:65282-1815-2 100 in 1 CARTON 01/01/2023 2 1 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 01/01/2023 Labeler - Spectra Medical Devices, LLC (118301171) Registrant - Spectra Medical Devices, LLC (118301171) Establishment Name Address ID/FEI Business Operations Safetec Of America Inc. 874965262 manufacture(65282-1815)