Label: BB MEDICATED PAIN RELIEVING OIL oil
-
Contains inactivated NDC Code(s)
NDC Code(s): 61079-014-13, 61079-025-13 - Packager: BB BIOCHEM LABORATORIES, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 1, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
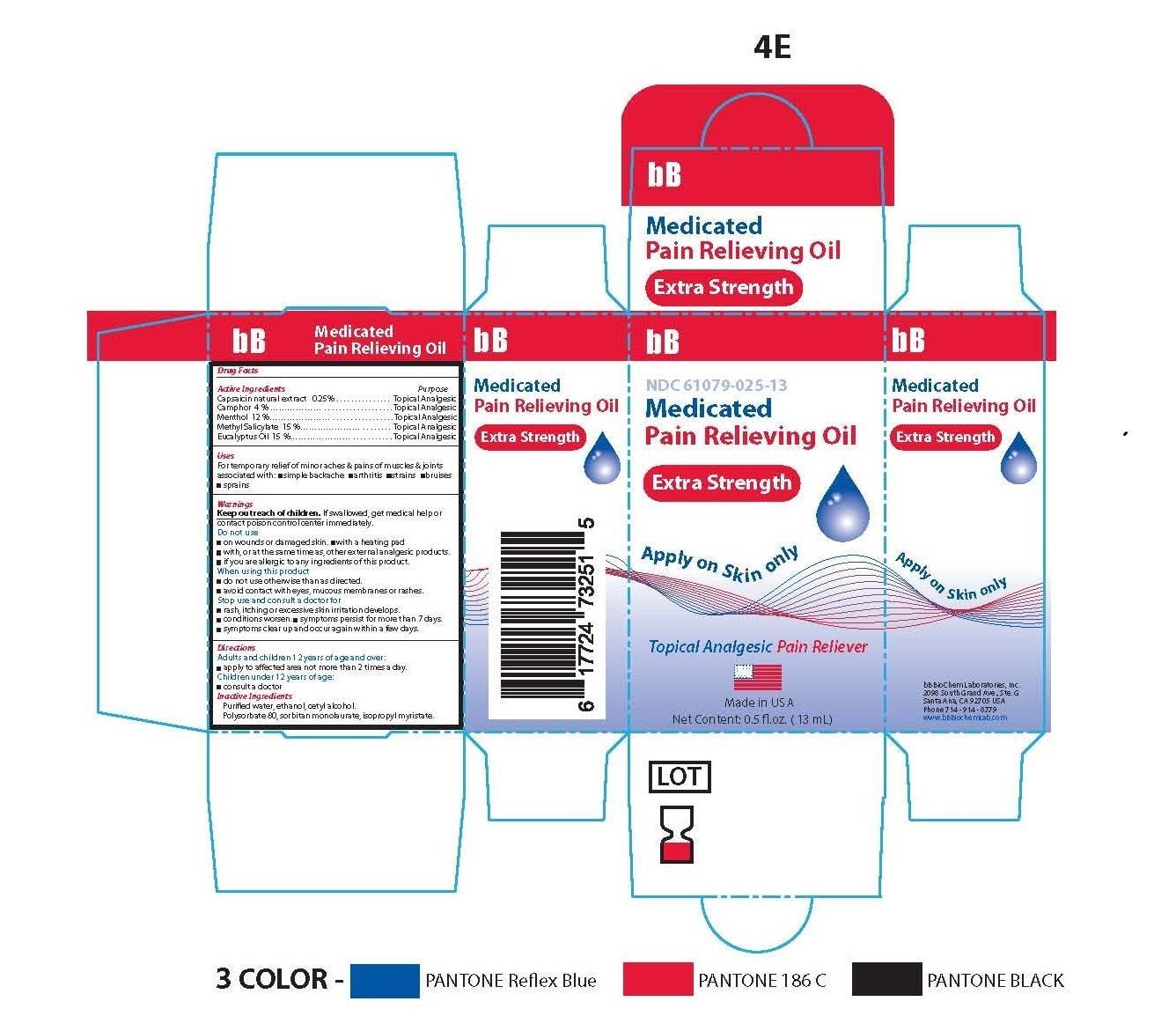
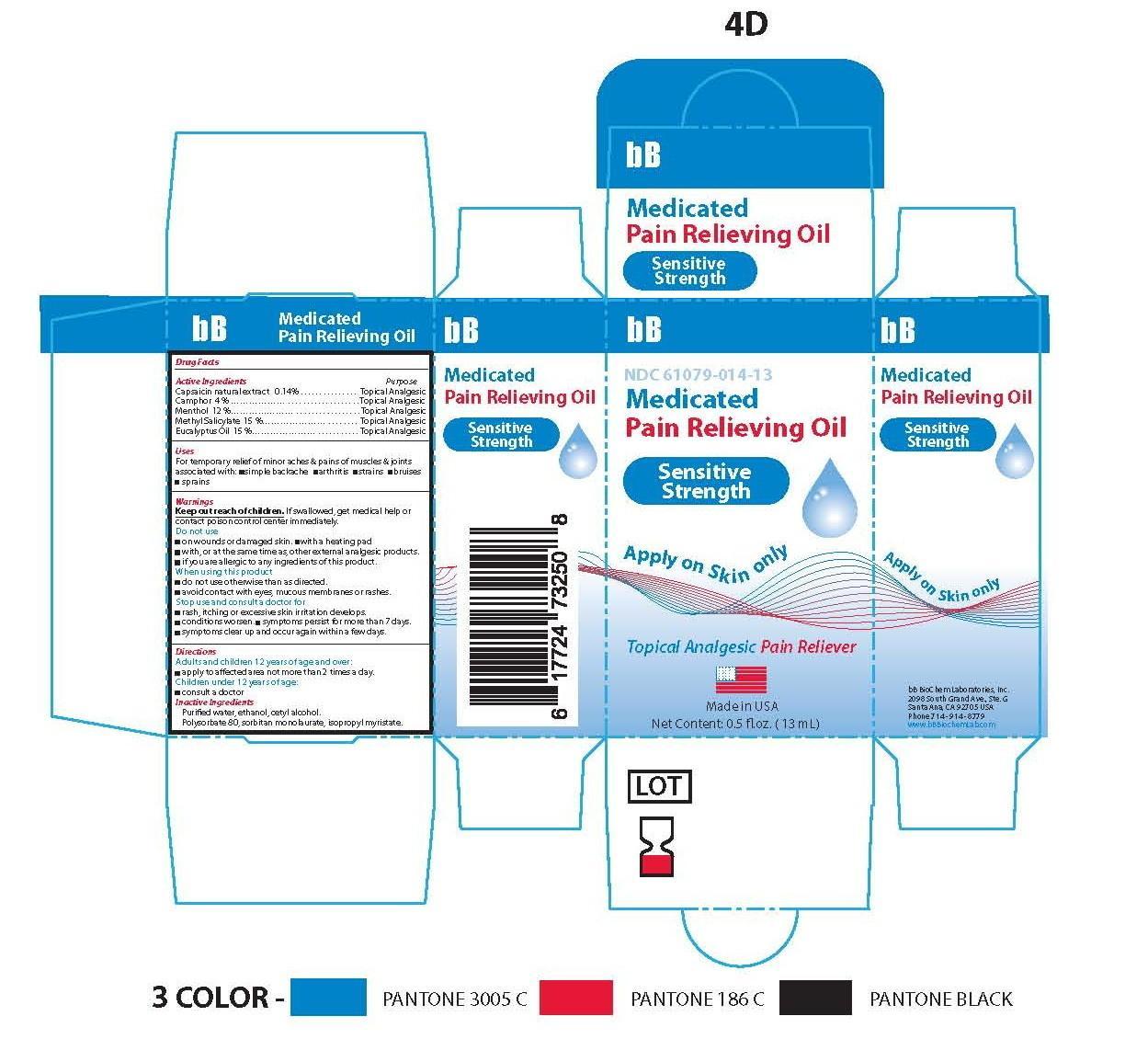
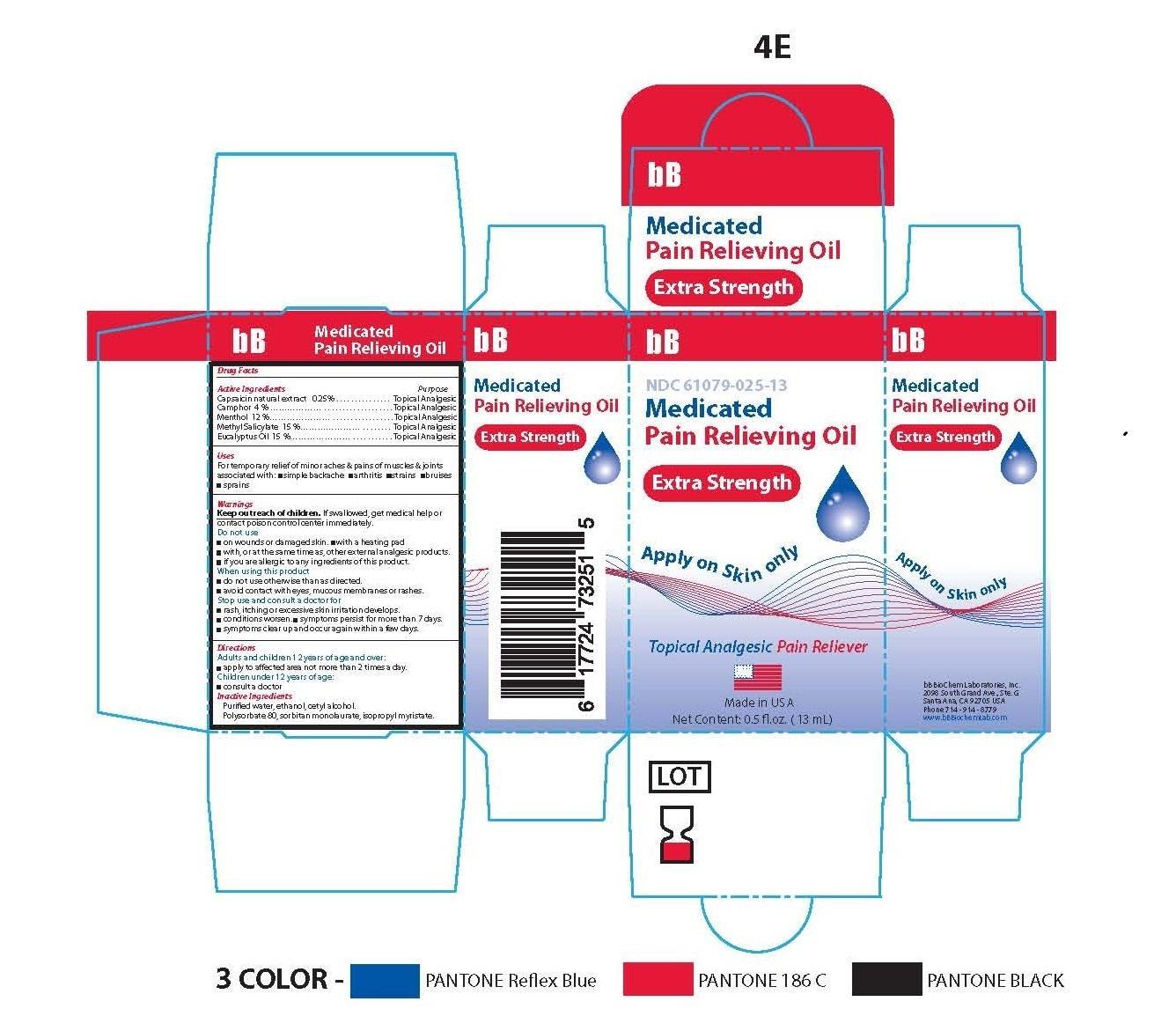
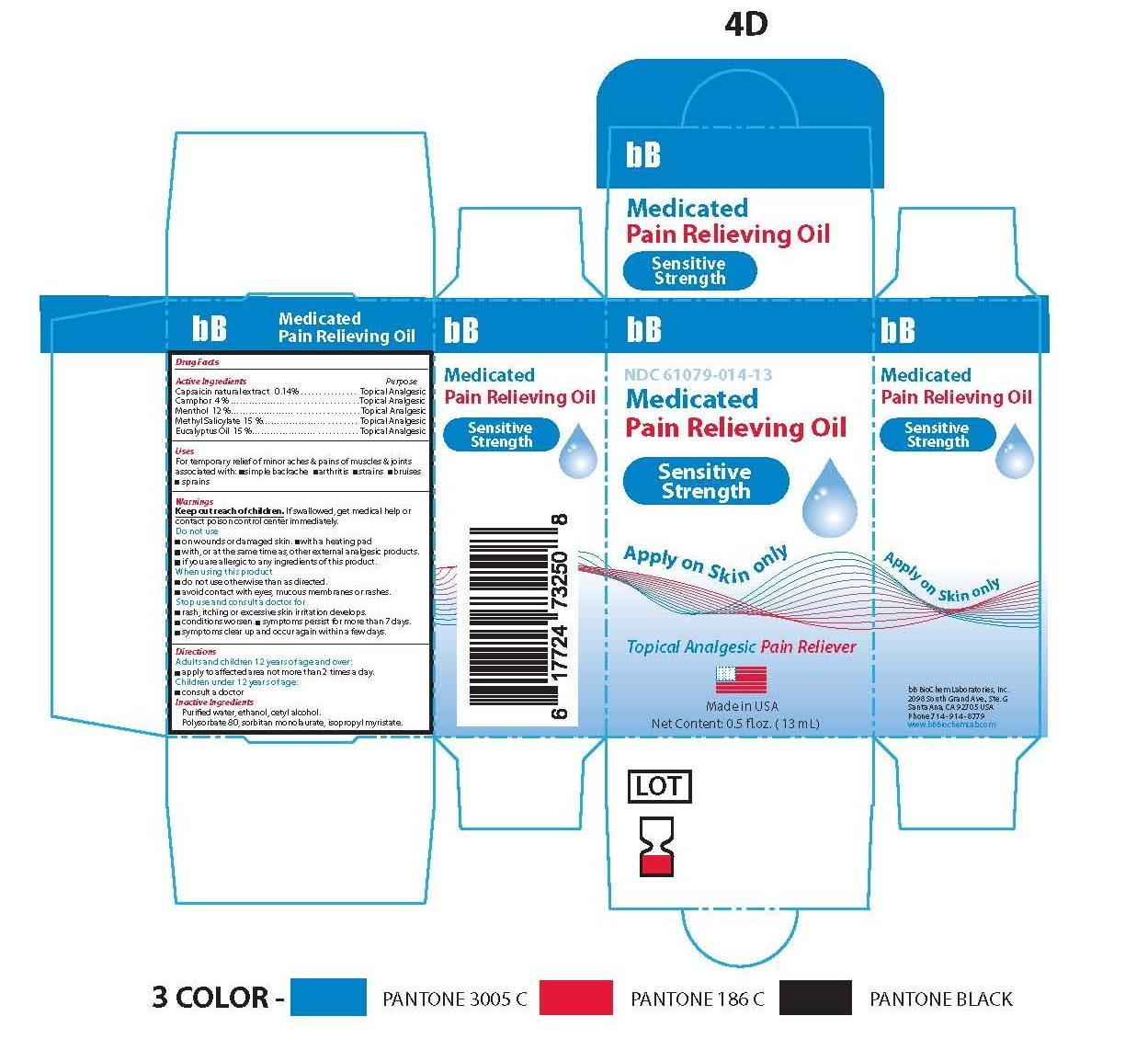
- Active Ingredients
-
Purpose
Capsaicin natural extract ........................................... .Topical Analgesic
Camphor …………………………………………….........Topical Analgesic
Menthol ....................................................................... Topical Analgesic
Methyl Salicylate ..........................................................Topical Analgesic
Eucalyptus Oil ..............................................................Topical Analgesic
- Uses
-
Warnings
Keep out reach of children
If swallowed, get medical help or contact poison control center immediately.
Do not use
■ on wounds or damaged skin.
■ with a heating pad
■ with, or at the same time as, other external analgesic products.
■ if you are allergic to any ingredients of this product.
When using this product:
■ do not use otherwise than as directed.
■ avoid contact with eyes, mucous membranes or rashes.
Stop use and consult a doctor for:
■ rash, itching or excessive skin irritation develops.
■ conditions worsen.
■ symptoms persist for more than7 days.
■ symptoms clear up and occur again within a few days.
If swallowed, get medical help or contact poison control center immediately.
- Directions and Allergy alert:
- Flammable and Safe Handling
- Do NOT use:
- When using this product:
- Stop use and ask a doctor if any of the following happens:
- Pregancy or Breast Feeding.
- Keep out of reach of children.
- Children under 12 years of age:
- Inactive Ingredients
-
Other Information
■ Due to its unique formulation, the solution can turn into different levels of white translucent, milky cloudiness at warmer temperatures; This is totally normal. Upon returning to cooler temperatures it should regain its typical transparency state.
■ Avoid storing product in direct sunlight.
■ Protect product from excessive moisture.
- Principle Display Panel-Extra Strength-Capsaicin 0.25/100
- Directions
- Principle Display Panel-Sensitive Strength-Capsaicin 0.14/100
-
INGREDIENTS AND APPEARANCE
BB MEDICATED PAIN RELIEVING OIL
bb medicated pain relieving oil oilProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61079-025 Route of Administration TOPICAL, PERCUTANEOUS, TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 3 g in 100 g LEVOMENTHOL (UNII: BZ1R15MTK7) (LEVOMENTHOL - UNII:BZ1R15MTK7) LEVOMENTHOL 12 g in 100 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 15 g in 100 g EUCALYPTUS OIL (UNII: 2R04ONI662) (EUCALYPTUS OIL - UNII:2R04ONI662) EUCALYPTUS OIL 15 g in 100 g CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN .25 g in 100 g Inactive Ingredients Ingredient Name Strength CETYL ALCOHOL (UNII: 936JST6JCN) 3 g in 100 g POLYSORBATE 80 (UNII: 6OZP39ZG8H) 11 g in 100 g SORBITAN MONOLAURATE (UNII: 6W9PS8B71J) 7 g in 100 g ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) 27 g in 100 g ALCOHOL (UNII: 3K9958V90M) 3 g in 100 g WATER (UNII: 059QF0KO0R) 4 g in 100 g Product Characteristics Color yellow (SLIGHT YELLOW COLORATION) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61079-025-13 13 g in 1 BOTTLE, GLASS 11/01/2014 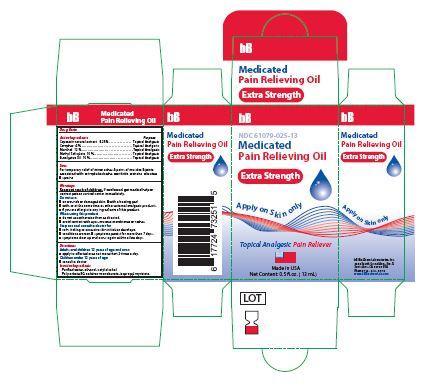
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 11/01/2014 BB MEDICATED PAIN RELIEVING OIL
bb medicated pain relieving oil oilProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61079-014 Route of Administration PERCUTANEOUS, TOPICAL, TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EUCALYPTUS OIL (UNII: 2R04ONI662) (EUCALYPTUS OIL - UNII:2R04ONI662) EUCALYPTUS OIL 15 g in 100 g CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN .14 g in 100 g CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 3 g in 100 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 15 g in 100 g LEVOMENTHOL (UNII: BZ1R15MTK7) (LEVOMENTHOL - UNII:BZ1R15MTK7) LEVOMENTHOL 12 g in 100 g Inactive Ingredients Ingredient Name Strength CETYL ALCOHOL (UNII: 936JST6JCN) 3 g in 100 g POLYSORBATE 80 (UNII: 6OZP39ZG8H) 11 g in 100 g SORBITAN MONOLAURATE (UNII: 6W9PS8B71J) 7 g in 100 g ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) 27 g in 100 g ALCOHOL (UNII: 3K9958V90M) 3 g in 100 g WATER (UNII: 059QF0KO0R) 4 g in 100 g Product Characteristics Color yellow (TRANSPARENT SLIGHT YELLOW COLORATION) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61079-014-13 13 g in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 11/01/2014 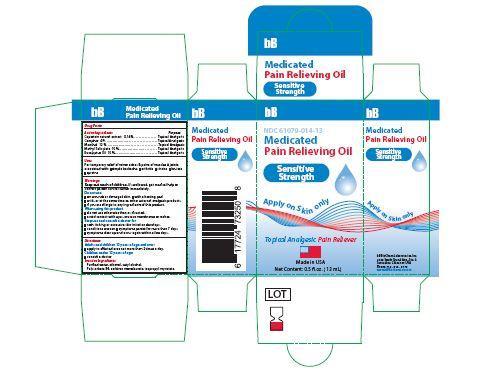
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 07/01/2014 Labeler - BB BIOCHEM LABORATORIES, INC. (053979073)