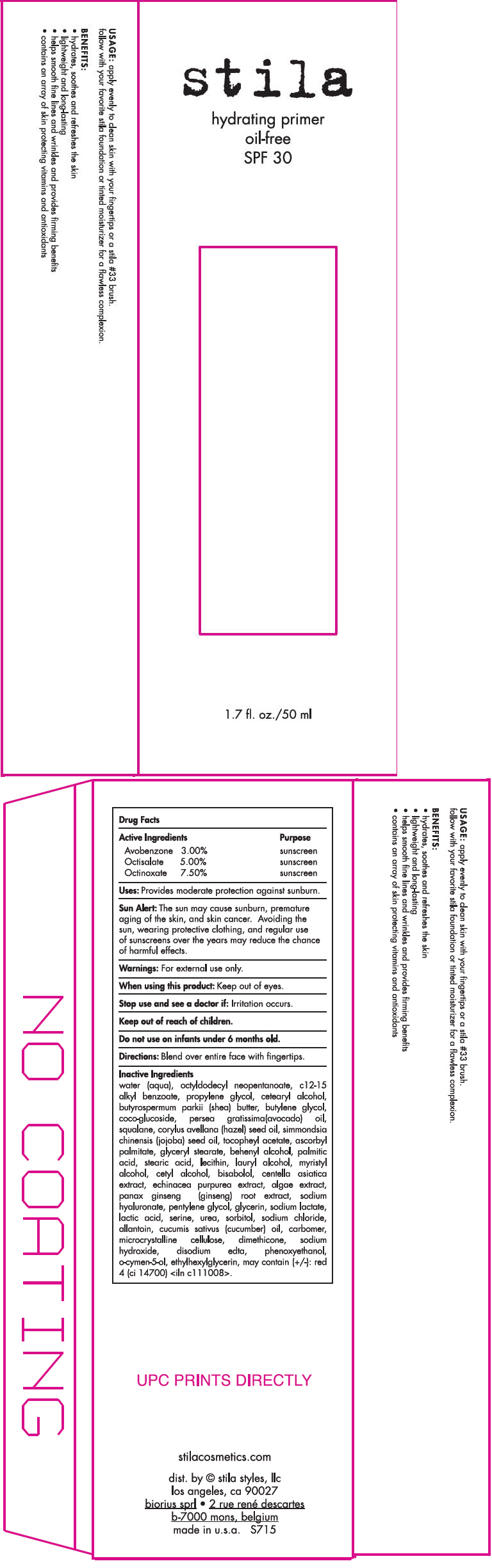
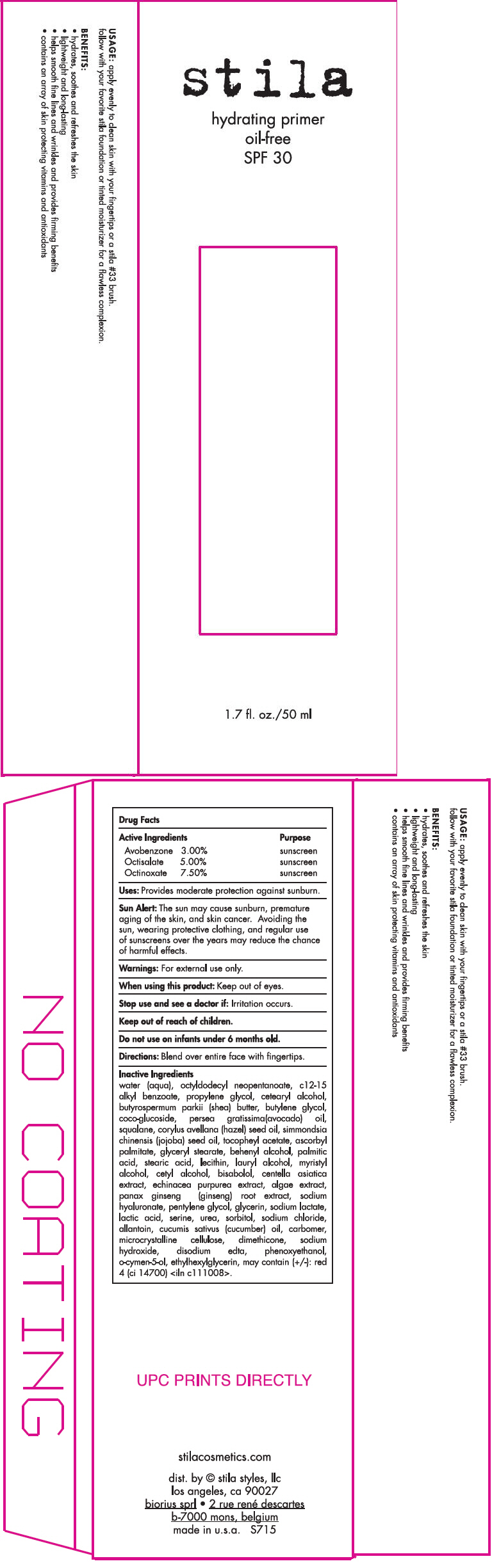
Label: STILA HYDRATING PRIMER OIL-FREE SPF 30 (ALL SHADES)- avobenzone, octisalate, and octinoxate lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 76049-715-01 - Packager: Stila Styles, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 18, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- ACTIVE INGREDIENT
- Uses
- Sun Alert
- Warnings
- Directions
-
Inactive Ingredients
water (aqua), octyldodecyl neopentanoate, c12-15 alkyl benzoate, propylene glycol, cetearyl alcohol, butyrospermum parkii (shea) butter, butylene glycol, coco-glucoside, persea gratissima(avocado) oil, squalane, corylus avellana (hazel) seed oil, simmondsia chinensis (jojoba) seed oil, tocopheyl acetate, ascorbyl palmitate, glyceryl stearate, behenyl alcohol, palmitic acid, stearic acid, lecithin, lauryl alcohol, myristyl alcohol, cetyl alcohol, bisabolol, centella asiatica extract, echinacea purpurea extract, algae extract, panax ginseng (ginseng) root extract, sodium hyaluronate, pentylene glycol, glycerin, sodium lactate, lactic acid, serine, urea, sorbitol, sodium chloride, allantoin, cucumis sativus (cucumber) oil, carbomer, microcrystalline cellulose, dimethicone, sodium hydroxide, disodium edta, phenoxyethanol, o-cymen-5-ol, ethylhexylglycerin, may contain (+/-): red 4 (ci 14700) <iln c111008>.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 ml Tube Carton
-
INGREDIENTS AND APPEARANCE
STILA HYDRATING PRIMER OIL-FREE SPF 30 (ALL SHADES)
avobenzone, octisalate, and octinoxate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76049-715 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 3 mg in 1 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 5 mg in 1 mL Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 7.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) octyldodecyl neopentanoate (UNII: X8725R883T) alkyl (c12-15) benzoate (UNII: A9EJ3J61HQ) propylene glycol (UNII: 6DC9Q167V3) cetostearyl alcohol (UNII: 2DMT128M1S) shea butter (UNII: K49155WL9Y) butylene glycol (UNII: 3XUS85K0RA) coco glucoside (UNII: ICS790225B) avocado oil (UNII: 6VNO72PFC1) squalane (UNII: GW89575KF9) european hazelnut oil (UNII: 8RQ2839AVG) jojoba oil (UNII: 724GKU717M) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) ascorbyl palmitate (UNII: QN83US2B0N) glyceryl monostearate (UNII: 230OU9XXE4) docosanol (UNII: 9G1OE216XY) palmitic acid (UNII: 2V16EO95H1) stearic acid (UNII: 4ELV7Z65AP) lauryl alcohol (UNII: 178A96NLP2) myristyl alcohol (UNII: V42034O9PU) cetyl alcohol (UNII: 936JST6JCN) levomenol (UNII: 24WE03BX2T) centella asiatica (UNII: 7M867G6T1U) echinacea purpurea (UNII: QI7G114Y98) asian ginseng (UNII: CUQ3A77YXI) hyaluronate sodium (UNII: YSE9PPT4TH) pentylene glycol (UNII: 50C1307PZG) glycerin (UNII: PDC6A3C0OX) sodium lactate (UNII: TU7HW0W0QT) lactic acid (UNII: 33X04XA5AT) serine (UNII: 452VLY9402) urea (UNII: 8W8T17847W) sorbitol (UNII: 506T60A25R) sodium chloride (UNII: 451W47IQ8X) allantoin (UNII: 344S277G0Z) cucumber seed oil (UNII: AKP926H71P) cellulose, microcrystalline (UNII: OP1R32D61U) dimethicone (UNII: 92RU3N3Y1O) sodium hydroxide (UNII: 55X04QC32I) edetate disodium (UNII: 7FLD91C86K) phenoxyethanol (UNII: HIE492ZZ3T) o-cymen-5-ol (UNII: H41B6Q1I9L) ethylhexylglycerin (UNII: 147D247K3P) fd&c red no. 4 (UNII: X3W0AM1JLX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76049-715-01 1 in 1 CARTON 1 50 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 08/30/2013 Labeler - Stila Styles, LLC (809192896) Establishment Name Address ID/FEI Business Operations MANA PRODUCTS 078870292 MANUFACTURE(76049-715)