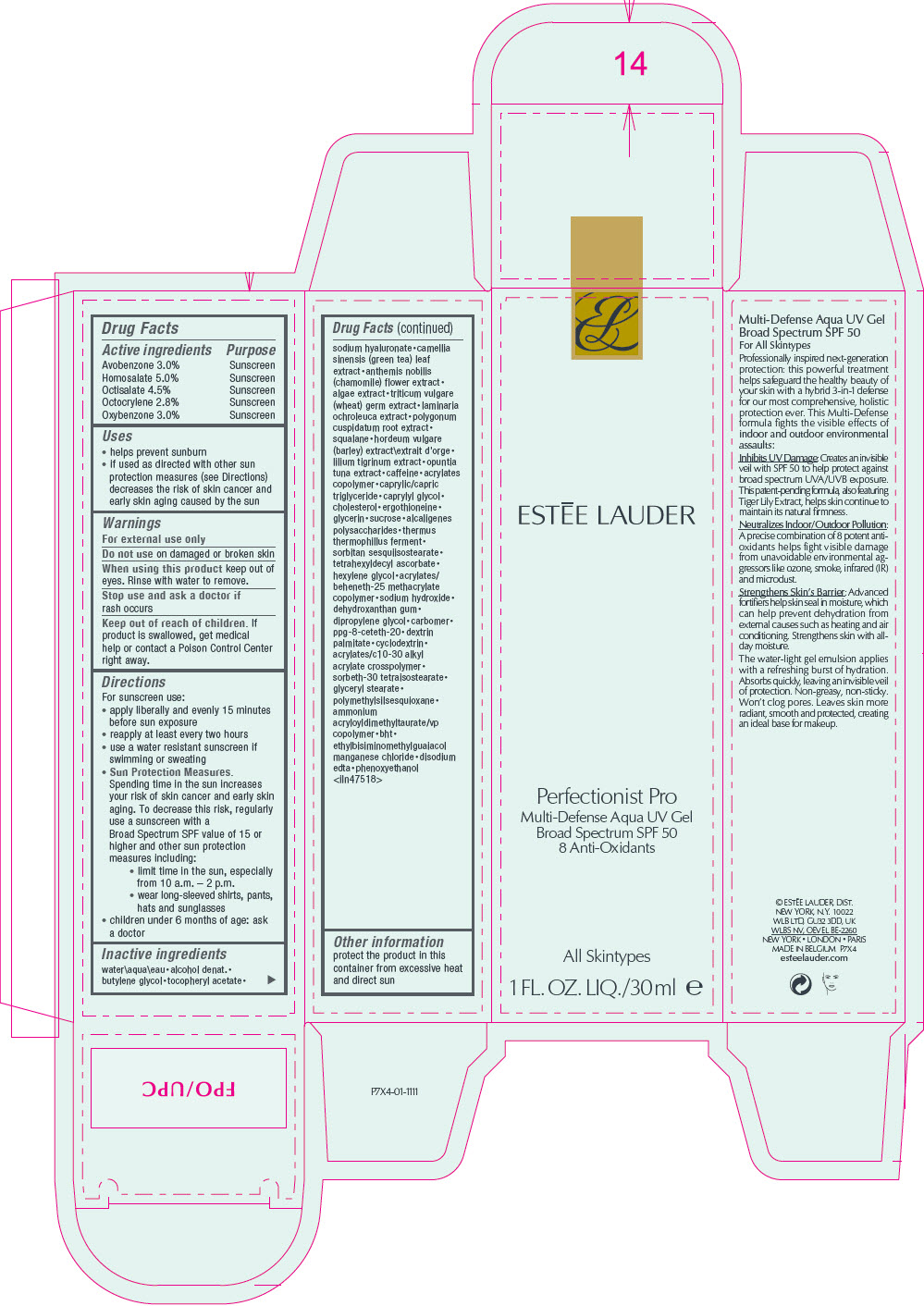
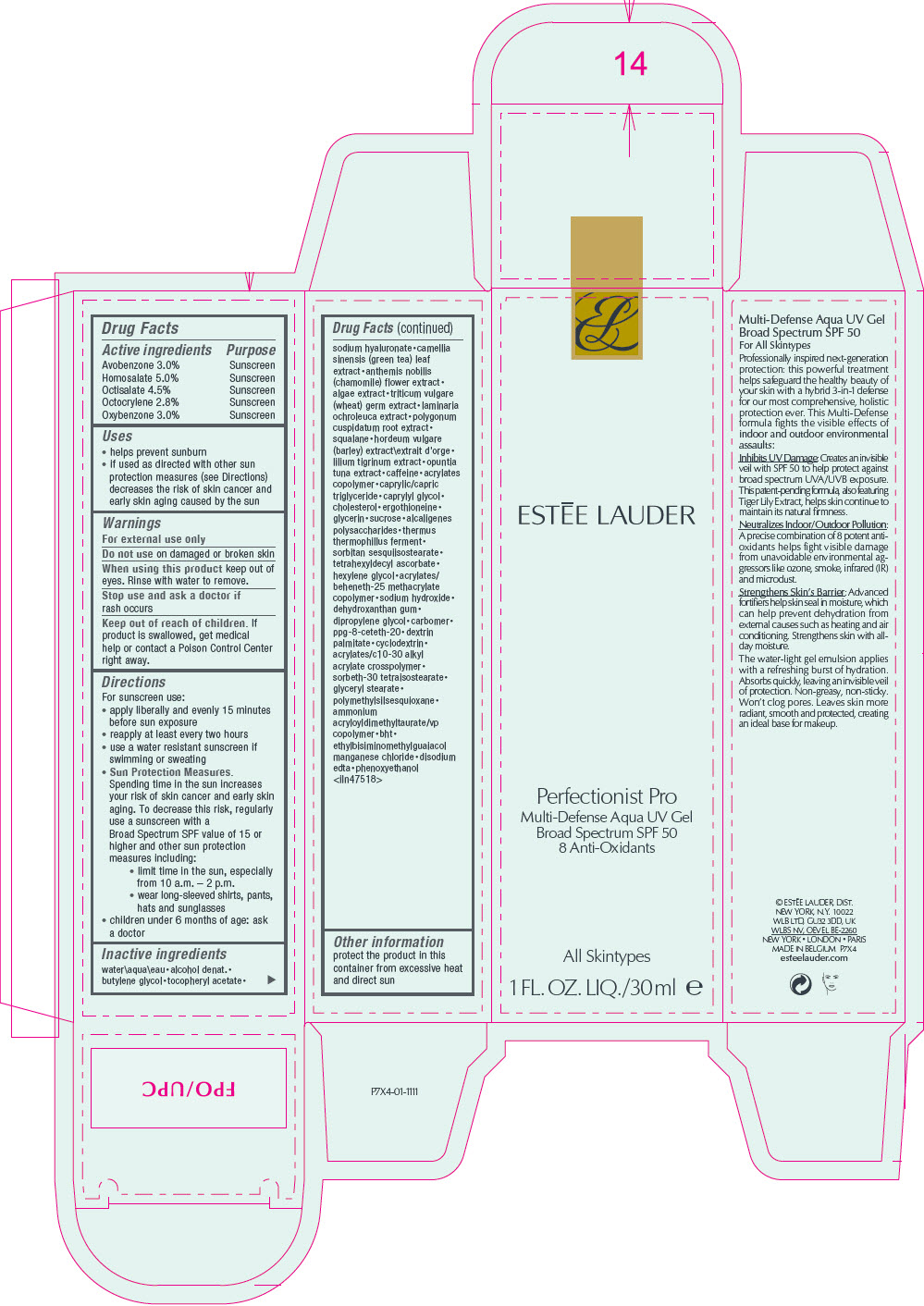
Label: ESTEE LAUDER MULTI-DEFENSE AQUA UV GEL BROAD SPECTRUM SPF 50- avobenzone, homosalate, octisalate, octocrylene, and oxybenzone lotion
- NDC Code(s): 11559-060-01
- Packager: ESTEE LAUDER INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions) decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply liberally and evenly 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive ingredients
water\aqua\eau • alcohol denat. • butylene glycol • tocopheryl acetate • sodium hyaluronate • camellia sinensis (green tea) leaf extract • anthemis nobilis (chamomile) flower extract • algae extract • triticum vulgare (wheat) germ extract • laminaria ochroleuca extract • polygonum cuspidatum root extract • squalane • hordeum vulgare (barley) extract\extrait d'orge • lilium tigrinum extract • opuntia tuna extract • caffeine • acrylates copolymer • caprylic/capric triglyceride • caprylyl glycol • cholesterol • ergothioneine • glycerin • sucrose • alcaligenes polysaccharides • thermus thermophillus ferment • sorbitan sesquiisostearate • tetrahexyldecyl ascorbate • hexylene glycol • acrylates/ beheneth-25 methacrylate copolymer • sodium hydroxide • dehydroxanthan gum • dipropylene glycol • carbomer • ppg-8-ceteth-20 • dextrin palmitate • cyclodextrin • acrylates/c10-30 alkyl acrylate crosspolymer • sorbeth-30 tetraisostearate • glyceryl stearate • polymethylsilsesquioxane • ammonium acryloyldimethyltaurate/vp copolymer • bht • ethylbisiminomethylguaiacol manganese chloride • disodium edta • phenoxyethanol
<iln47518> - Other information
- PRINCIPAL DISPLAY PANEL - 30 ml Tube Carton
-
INGREDIENTS AND APPEARANCE
ESTEE LAUDER MULTI-DEFENSE AQUA UV GEL BROAD SPECTRUM SPF 50
avobenzone, homosalate, octisalate, octocrylene, and oxybenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11559-060 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 50 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 28 mg in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ETHYLBISIMINOMETHYLGUAIACOL MANGANESE CHLORIDE (UNII: SM5YJ88LTU) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) 1,2-BUTANEDIOL (UNII: RUN0H01QEU) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CHAMAEMELUM NOBILE FLOWER (UNII: O2T154T6OG) SARGASSUM FILIPENDULA (UNII: 55P66J5H7N) WHEAT GERM (UNII: YR3G369F5A) LAMINARIA OCHROLEUCA (UNII: 4R2124HE76) REYNOUTRIA JAPONICA ROOT (UNII: 7TRV45YZF7) SQUALANE (UNII: GW89575KF9) HORDEUM VULGARE ROOT (UNII: 790S39483Z) LILIUM LANCIFOLIUM BULB (UNII: 47Z05W73EZ) OPUNTIA TUNA WHOLE (UNII: DI94T5T0ZY) CAFFEINE (UNII: 3G6A5W338E) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHOLESTEROL (UNII: 97C5T2UQ7J) ERGOTHIONEINE (UNII: BDZ3DQM98W) GLYCERIN (UNII: PDC6A3C0OX) SUCROSE (UNII: C151H8M554) ALCALIGENES FAECALIS (UNII: 05KB30NGW2) THERMUS THERMOPHILUS LYSATE (UNII: 775R692494) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) HEXYLENE GLYCOL (UNII: KEH0A3F75J) BEHENETH-25 METHACRYLATE (UNII: 108R05PWG6) SODIUM HYDROXIDE (UNII: 55X04QC32I) DEHYDROXANTHAN GUM (UNII: 63ZP7I1BQO) DIPROPYLENE GLYCOL (UNII: E107L85C40) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) PPG-2-CETETH-9 (UNII: 1OTA54V264) DEXTRIN PALMITATE (CORN; 20000 MW) (UNII: 89B2BSF9I3) BETADEX (UNII: JV039JZZ3A) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) PEG-10 SORBITAN LAURATE (UNII: 4Z93U4C2WN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11559-060-01 1 in 1 CARTON 05/08/2020 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 05/04/2020 Labeler - ESTEE LAUDER INC (005914387) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations Estee Lauder N.V. 370151326 manufacture(11559-060) , pack(11559-060) , label(11559-060)