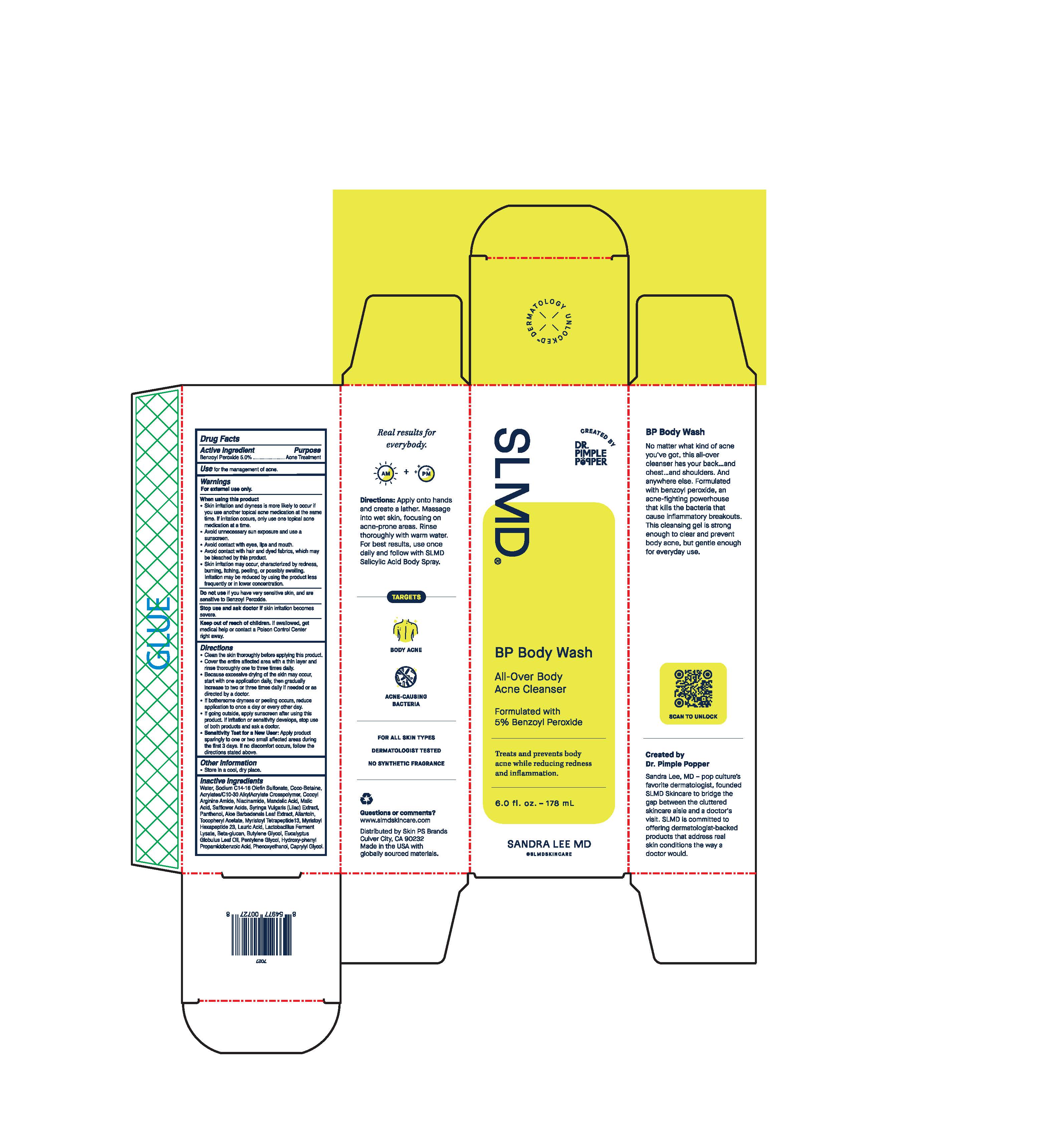
Label: BP BODY WASH- body wash gel
- NDC Code(s): 73318-7005-1
- Packager: Skin PS Brands
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
-
When using this product
- Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- avoid unnecessary sun exposure and use a sunscreen.
- avoid contact with eyes, lips and mouth.
- avoid contact with hair and dyed fabrics, which may be bleached by this product.
- Skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
- Do not use
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
- Clean the skin thoroughly before applying this product.
- Cover the entire affected area with a thin layer and rinse thoroughly one to three times daily.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three daily if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- If going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
- Sensitivity Test for a New User: Apply product sparingly to one or two small affected areas during the first three days. if no discomfort occurs, follow the directions stated above.
- Other Information
-
Inactive Ingredients
Water, Sodium C14-15 Olefin Sulfonate, Coco-Betaine, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Cocoyl Arginine Amide, Niacinamide, Mandelic Acid, Malic Acid, Safflower Acids, Syringa Vulgaris (Lilac) Extract, Panthenol, Aloe Barbadensis Leaf Extract, Allantoin, Tocopheryl Acetate, Myristoyl Tetrapeptide 13, Myristoyl Hexapeptide 23, Lauric Acid, Lactobacillus Ferment Lysate, Beta-glucan, Butylene Glycol, Eucalyptus Globulus Leaf Oil, Pentylene Glycol, Hydroxy-phenyl Propamidobenzoic Acid, Phenoxyethanol, Caprylyl Glycol.
- Questions or comments?
-
SLMD
Sandra Lee MD
Created by Dr. Pimple Popper
BP Body Wash
All-Over Body
Acne Cleanser
Formulated with
5% Benzoyl Peroxide
Treats and prevents body acne while reducing redness and inflammation.
6.0 fl. oz. - 178 mL
SANDRA LEE MD
@SLMDSKINCARE
Unit Carton:
 Primary:
Primary:

Real results for everybody.
AM + PM
Directions: Apply onto hands and create a lather. Massage into wet skin, focusing on acne-prine areas. Rinse thoroughly with warm water. For best results, use once daily and follow with SLMD Salicylic Acid Body Spray.
TARGETS
BODY ACNE
ACNE-CAUSING BACTERIA
FOR ALL SKIN TYPES
DERMATOLOGIST TESTED
NO SYNTHETIC FRAGRANCE
Questions or comments?
www.slmdskincare.com
Distributed by Skin PS Brands
Culver City, CA 90232
Made in the USA with globally sourced materials.
BP Body Wash
No matter what kind of acne you've got, this all-over cleanser has your back..and chest..and shoulders. And anywhere else. Formulated with benzoyl peroxide, an acne-fighting powerhouse that kills the bacteria that cause inflammatory breakouts. This cleansing gel is strong enough to clear and prevent body acne, but gentle enough for everyday use.
Created by Dr. Pimple Popper
Sandra Lee, MD - pop culture's favorite dermatologist, founded SLMD Skincare to bridge the gap between the cluttered skincare aisle and a doctor's visit. SLMD is committed to offering dermatologist-backed products that address real skin conditions the way a doctor would.
-
INGREDIENTS AND APPEARANCE
BP BODY WASH
body wash gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73318-7005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) ARGININE (UNII: 94ZLA3W45F) NIACINAMIDE (UNII: 25X51I8RD4) MANDELIC ACID (UNII: NH496X0UJX) MALIC ACID (UNII: 817L1N4CKP) SAFFLOWER ACID (UNII: Y788KS82OV) SYRINGA VULGARIS WHOLE (UNII: U49SHU1VCI) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) ALLANTOIN (UNII: 344S277G0Z) HYDROXYPHENYL PROPAMIDOBENZOIC ACID (UNII: 25KRT26H77) EUCALYPTUS OIL (UNII: 2R04ONI662) PANTHENOL (UNII: WV9CM0O67Z) SODIUM CARBOXYMETHYL .BETA.-GLUCAN (DS 0.65-0.85) (UNII: 2YGO1190AP) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LIMOSILACTOBACILLUS FERMENTUM (UNII: 2C1F12C6AP) LAURIC ACID (UNII: 1160N9NU9U) MYRISTOYL HEXAPEPTIDE-12, (VAL-GLY-VAL-ALA-PRO-GLY)- (UNII: ZWF521YI75) MYRISTOYL TETRAPEPTIDE-4 (UNII: 87AV1IB2EU) COCO-BETAINE (UNII: 03DH2IZ3FY) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73318-7005-1 1 in 1 CARTON 07/01/2023 1 178 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 07/01/2023 Labeler - Skin PS Brands (081085221) Registrant - Skin PS Brands (081085221) Establishment Name Address ID/FEI Business Operations Owen Biosciences 790003045 manufacture(73318-7005)