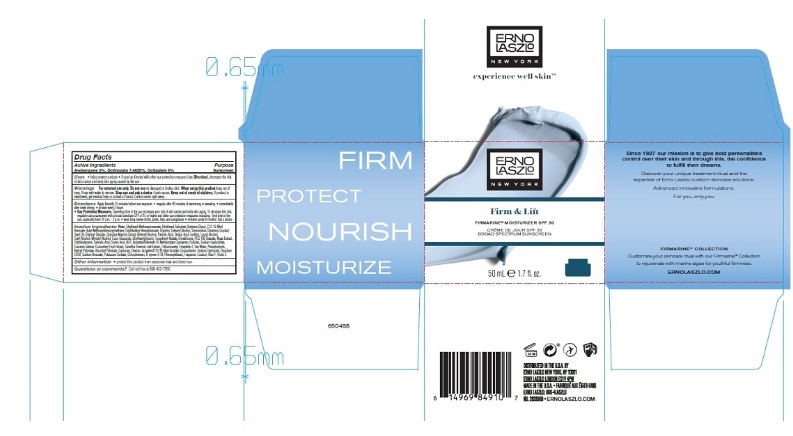
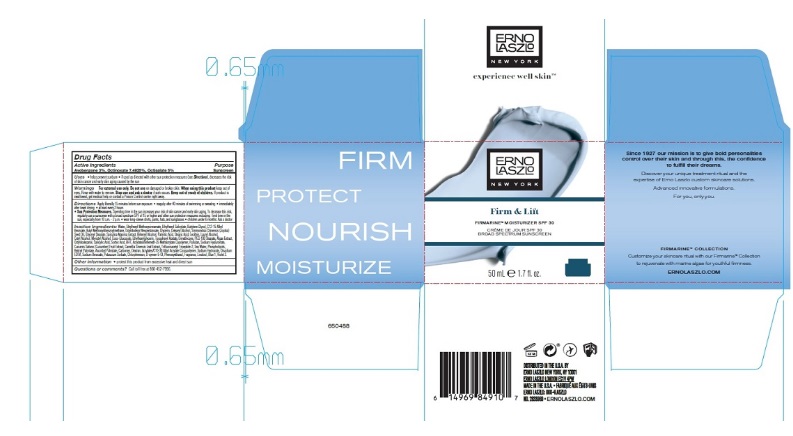
Label: FIRMARINE MOISTURIZER SPF 30- avobenzone, octinoxate, and octisalate lotion
- NDC Code(s): 57913-2833-0
- Packager: Erno Laszlo, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
Apply liberally 15 minutes before sun exposure
- reapply: after 40 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
-
Sun Protection Measures.Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
-
Inactive Ingredients
Water, Butylene Glycol, Octyldodecyl Neopentanoate, C12-15 Alkyl Benzoate, Glycerin, Simmondsia Chinensis (Jojoba) Seed Oil, Glyceryl Stearate, Cetearyl Alcohol, Behenyl Alcohol, Palmitic Acid, Stearic Acid, Lecithin, Lauryl Alcohol, Cetyl Alcohol, Myristyl Alcohol, Coco-Glucoside, Ethylhexylglycerin, Dimethicone, PEG-100 Stearate, Spirulina Maxima Extract, Cucumis Sativus (Cucumber) Fruit Extract, Camellia Sinensis Leaf Extract, Phospholipids, Tocopheryl Acetate, Retinyl Palmitate, Ascorbyl Palmitate, Algae Extract, Pullulan, Sodium Hyaluronate, Trifluoroacetyl Tripeptide-2, Sea Water, Carbomer, Dextran, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Sodium Hydroxide, Disodium EDTA, O-Cymen-5-Ol, Phenoxyethanol, Fragrance, Blue 1, Ext Violet 2.
- Other information
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- Product label
-
INGREDIENTS AND APPEARANCE
FIRMARINE MOISTURIZER SPF 30
avobenzone, octinoxate, and octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57913-2833 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) GLYCERIN (UNII: PDC6A3C0OX) JOJOBA OIL (UNII: 724GKU717M) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DOCOSANOL (UNII: 9G1OE216XY) PALMITIC ACID (UNII: 2V16EO95H1) STEARIC ACID (UNII: 4ELV7Z65AP) LAURYL ALCOHOL (UNII: 178A96NLP2) CETYL ALCOHOL (UNII: 936JST6JCN) MYRISTYL ALCOHOL (UNII: V42034O9PU) COCO GLUCOSIDE (UNII: ICS790225B) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) DIMETHICONE (UNII: 92RU3N3Y1O) PEG-100 STEARATE (UNII: YD01N1999R) SPIRULINA MAXIMA (UNII: 9K7IG15M0Q) CUCUMBER (UNII: YY7C30VXJT) GREEN TEA LEAF (UNII: W2ZU1RY8B0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ASCORBYL PALMITATE (UNII: QN83US2B0N) PULLULAN (UNII: 8ZQ0AYU1TT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) EDETATE DISODIUM (UNII: 7FLD91C86K) O-CYMEN-5-OL (UNII: H41B6Q1I9L) PHENOXYETHANOL (UNII: HIE492ZZ3T) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) EXT. D&C VIOLET NO. 2 (UNII: G5UX3K0728) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57913-2833-0 1 in 1 CARTON 04/10/2013 1 50 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/10/2013 Labeler - Erno Laszlo, Inc. (098821031) Establishment Name Address ID/FEI Business Operations Mana Products 078870292 manufacture(57913-2833)