Label: DOCUSATE SODIUM capsule, liquid filled
- NDC Code(s): 0615-8041-30, 0615-8041-39
- Packager: NCS HealthCare of KY, LLC dba Vangard Labs
- This is a repackaged label.
- Source NDC Code(s): 45802-486
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each softgel)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
Ask a doctor or pharmacist before use if you are
taking any other drug. Take this product two or more hours before or after other drugs. Laxatives may affect how other drugs work.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
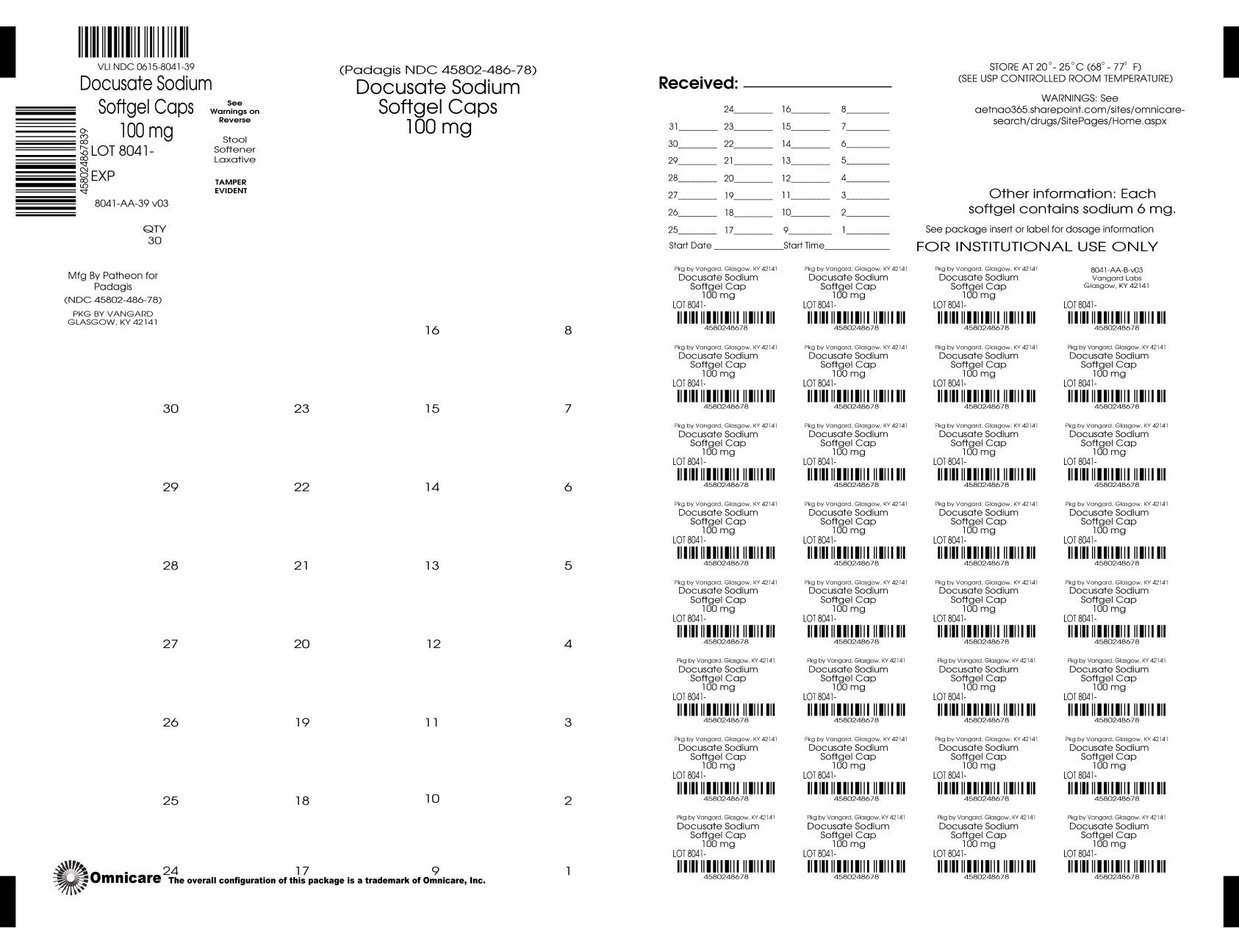
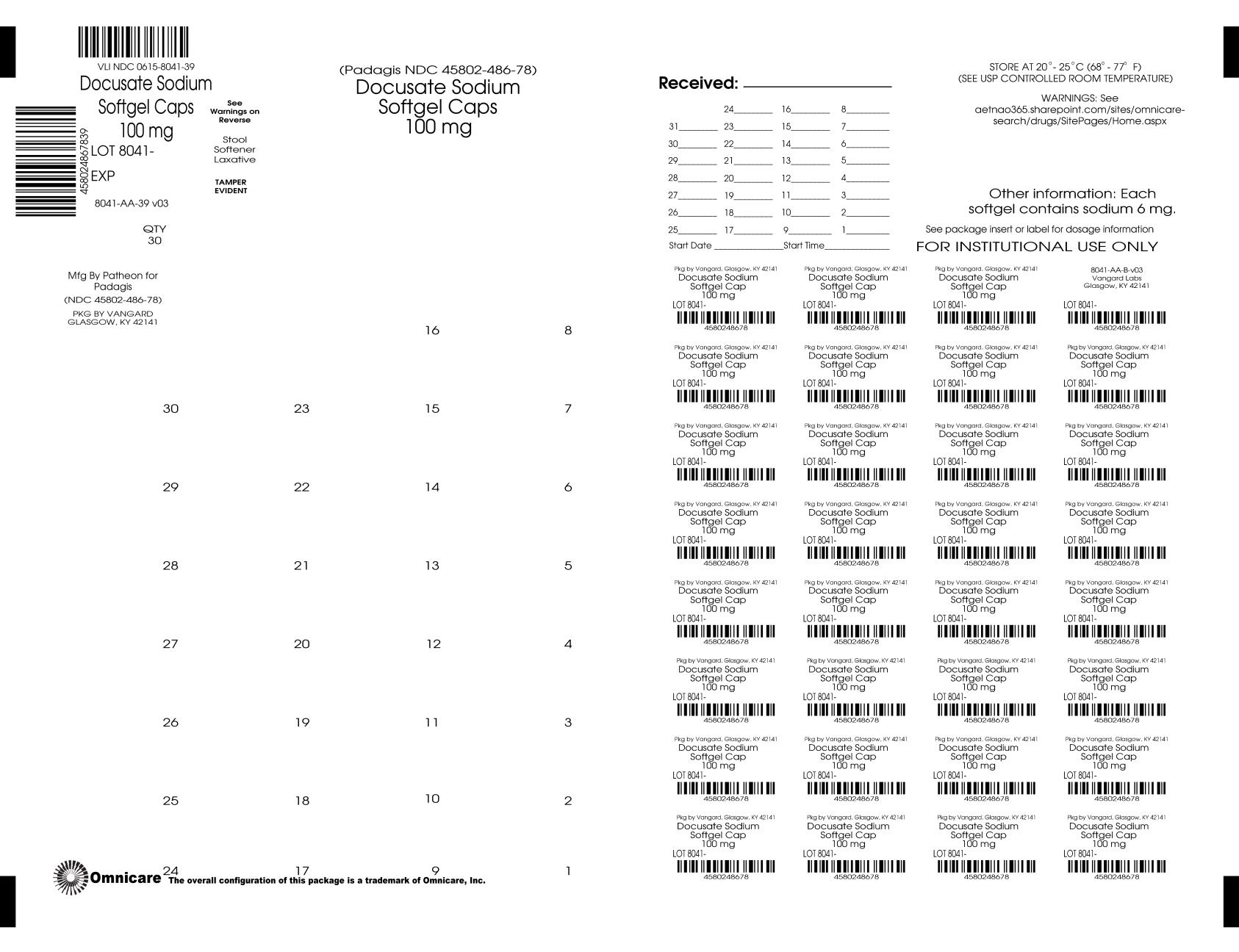
- Principal Display Panel - BINGO CARD
- Principal Display Panel - UNIT DOSE
-
INGREDIENTS AND APPEARANCE
DOCUSATE SODIUM
docusate sodium capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0615-8041(NDC:45802-486) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 100 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) WATER (UNII: 059QF0KO0R) SORBITAN (UNII: 6O92ICV9RU) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics Color red, white (to off beige) Score no score Shape OVAL (softgel) Size 13mm Flavor Imprint Code L486 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0615-8041-39 30 in 1 BLISTER PACK; Type 0: Not a Combination Product 04/14/2016 2 NDC:0615-8041-30 6 in 1 BOX, UNIT-DOSE 04/14/2016 2 5 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 10/28/2008 Labeler - NCS HealthCare of KY, LLC dba Vangard Labs (050052943) Establishment Name Address ID/FEI Business Operations NCS HealthCare of KY, LLC dba Vangard Labs 050052943 repack(0615-8041)