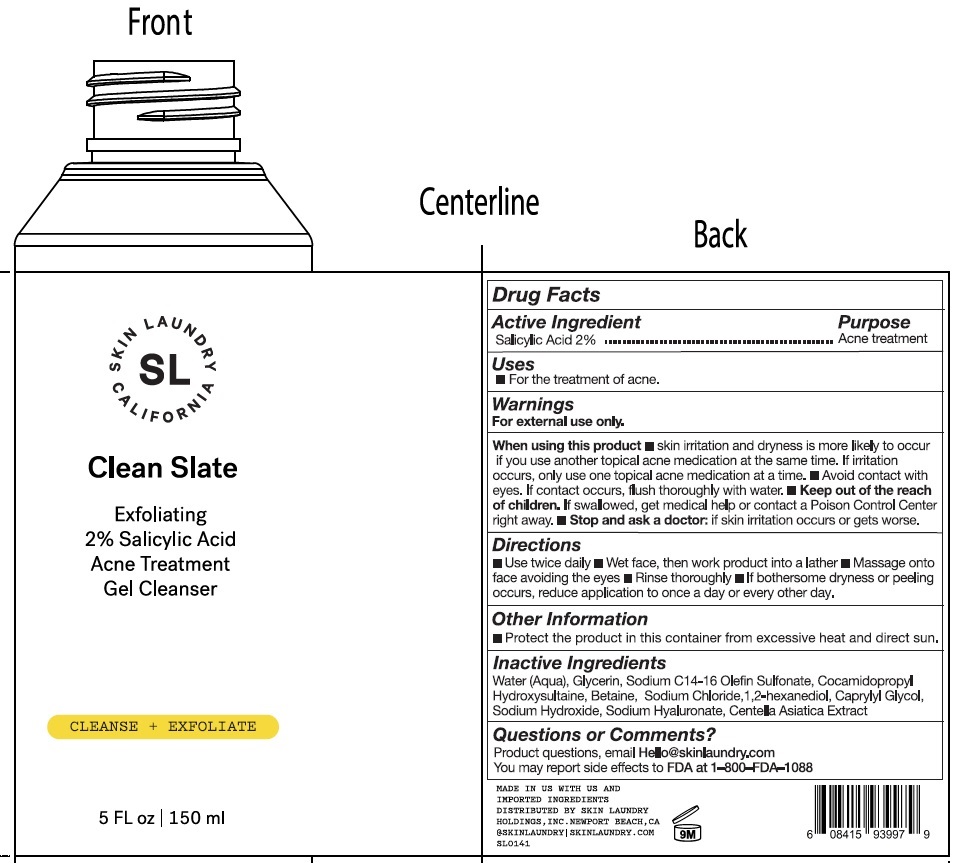
Label: SKIN LAUNDRY. CLEAN SLATE EXFOLIATING 2 SALICYLIC ACID TREATMENT GEL CLEANSER CLEANSE EXFOLIATE- salicylic acid gel
- NDC Code(s): 83727-294-00
- Packager: Skin Laundry Holdings, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
-
Warnings
For external use only.
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- Avoid contact with eyes. If contact occurs, flush thoroughly with water.
- Directions
- Other Information
- Inactive Ingredients
- Questions or Comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
SKIN LAUNDRY. CLEAN SLATE EXFOLIATING 2 SALICYLIC ACID TREATMENT GEL CLEANSER CLEANSE EXFOLIATE
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83727-294 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) COCAMIDOPROPYL HYDROXYSULTAINE (UNII: 62V75NI93W) BETAINE (UNII: 3SCV180C9W) SODIUM CHLORIDE (UNII: 451W47IQ8X) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CENTELLA ASIATICA TRITERPENOIDS (UNII: 4YS74Q4G4J) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83727-294-00 150 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/14/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 10/14/2023 Labeler - Skin Laundry Holdings, Inc. (118584348)