Label: VETSTARCH- hydroxyethyl starch 130/0.4 substitution injection, solution
- NDC Code(s): 54771-4950-2, 54771-4950-5
- Packager: Zoetis Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated July 15, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
HIGHLIGHTS OF PRESCRIBING INFORMATION:
These highlights do not include all of the information needed to use VetStarch safely and effectively. See full prescribing information for VetStarch.
VetStarch™
(6% HYDROXYETHYL STARCH 130/0.4 IN 0.9% SODIUM CHLORIDE INJECTION)
For administration by intravenous infusion.INDICATIONS AND USAGE:
VetStarch is a plasma volume substitute indicated for the treatment and prophylaxis of hypovolemia.DOSAGE AND ADMINISTRATION:
Administer by intravenous infusion only.
•Daily dose and rate of infusion depend on the patient's blood loss, hemodynamics and on the hemodilution effects
•Initiate infusion slowly due to possible anaphylactoid reactionsDOSAGE FORMS AND STRENGTHS:
250 ml and 500 ml flexible plastic intravenous solution containers. Each 100 ml contains 6 g hydroxyethyl starch 130/0.4 in Isotonic sodium chloride injection.CONTRAINDICATIONS:
•Known hypersensitivity to hydroxyethyl starch
•Fluid overload e.g., pulmonary edema and congestive heart failure
•Renal failure with oliguria or anuria not related to hypovolemia
•Patients receiving dialysis
•Severe hypernatremia or severe hyperchloremia
•Intracranial bleedingWARNINGS AND PRECAUTIONS:
•Anaphylactoid and hypersensitivity reactions
•Avoid fluid overload; adjust dosage in patients with cardiac or renal dysfunction
•In severe dehydration, a crystalloid solution should be given first
•Observe caution in patients with severe liver disease or bleeding disorders
•Monitor kidney function, fluid balance and serum electrolytes
•In humans elevated serum amylase values may occur and interfere with the diagnosis of pancreatitis
•High dosages may cause dilution of blood componentsADVERSE REACTIONS:
•Anaphylactoid/hypersensitivity reactions can occur. Most common adverse reactions ar pruritus, hemodilution (resulting in dilution of blood components, e.g., coagulation factors and other plasma proteins, and in a decrease in hematocrit).To report SUSPECTED ADVERSE REACTIONS, call Zoetis Inc. at 1-888-963-8471.
DRUG INTERACTIONS:
No interactions with other drugs or nutritional products are known.
The safety and compatibility of additives have not been established.USE IN SPECIFIC POPULATIONS:
Renal impaired: Use care in dosage selection.After reviewing the Highlights Section, please read the following full prescribing information for this drug.
Revised: July 2015
01-59-22-002C
-
FULL PRESCRIBING INFORMATION: CONTENTS
2. DOSAGE AND ADMINISTRATION
2.1 Adult Dose
2.2 Directions for Use of VetStarch™5. WARNINGS AND PRECAUTIONS
5.1 General Warnings and Precautions
5.2 Monitoring: Laboratory Tests
5.3 Interference with Laboratory Tests6. ADVERSE REACTIONS
6.1 Overall Adverse Reaction Profile8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Renal Impairment11. CLINICAL PHARMACOLOGY
11.1 Mechanism of Action12. NONCLINICAL TOXICOLOGY
12.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
12.2 Animal Toxicology and Pharmacology
12.2.1 Toxicology
12.2.2 Pharmacology14. HOW SUPPLIED / STORAGE AND HANDLING
FULL PRESCRIBING INFORMATION
For Animal Use Only
CAUTION: Federal Law (USA) restricts this drug to use by or on the order of a licensed veterinarian. - 1. INDICATIONS AND USAGE
-
2. DOSAGE AND ADMINISTRATION
VetStarch is administered by intravenous infusion only. The daily dose and rate of infusion depend on the patient’s blood loss, on the maintenance or restoration of hemodynamics and on the hemodilution (dilution effect). VetStarch can be administered repetitively over several days. [See Warnings and Precautions (5)]
The initial 10 to 20 mL should be infused slowly, keeping the patient under close observation due to possible anaphylactoid reactions. [see General Warnings and Precautions (5.1)]2.1 Adult Dose
As a general recommendation, the class of synthetic colloids are prescribed at doses up to 20 mL per kg of body weight per day in small animal patients.1 In a 30 kg patient, this is a dose of 600 mL of VetStarch (equivalent to 1.2 g hydroxyethyl starch and 3.1 mEq sodium per kg of body weight). The use of 6% hydroxyethyl starch 130/0.4 has been dosed at 50 mL per kg of body weight per day in human studies.2
2.2 Directions for Use of VetStarch
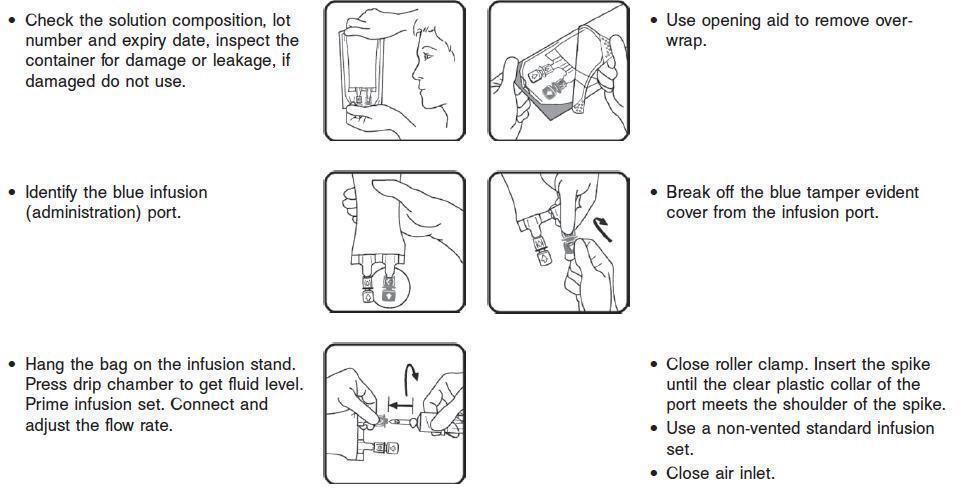
1. Do not remove the IV container from its overwrap until immediately before use.
2. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
3. Do not administer unless the solution is clear, free from particles and the IV container is undamaged.
4. VetStarch should be used immediately after insertion of the administration set.
5. Do not vent.
6. If administered by pressure infusion, air should be withdrawn or expelled from the bag through the medication/administration port prior to infusion.
7. Discontinue the infusion if an adverse reaction occurs.
8. It is recommended that administration sets be changed at least once every 24 hours.
9. For single use only. Discard unused portion.INCOMPATIBILITIES
The safety and compatibility of additives have not been established.
- 3. DOSAGE FORMS AND STRENGTHS
-
4. CONTRAINDICATIONS
The use of VetStarch™ is contraindicated in the following conditions:
• Known hypersensitivity to hydroxyethyl starch [see General Warnings and Precautions (5.1)]
• Fluid overload (hyperhydration) and especially in cases of pulmonary edema and congestive heart failure
• Renal failure with oliguria or anuria not related to hypovolemia
• Patients receiving dialysis treatment
• Severe hypernatremia or severe hyperchloremia
• Intracranial bleeding -
5. WARNINGS AND PRECAUTIONS
5.1 General Warnings and Precautions
Anaphylactoid reactions (bradycardia, tachycardia, bronchospasm, non-cardiac pulmonary edema) have been reported with solutions containing hydroxyethyl starch.3 If a hypersensitivity reaction occurs, administration of the drug should be discontinued immediately and the appropriate treatment and supportive measures should be undertaken until symptoms have resolved. [see Adverse Reactions (6)]
Fluid status and rate of infusion should be assessed regularly during treatment, especially in patients with cardiac insufficiency or severe kidney dysfunction.
In cases of severe dehydration, a crystalloid solution should be given first. Generally, sufficient fluid should be administered in order to avoid dehydration.
Caution should be observed before administering VetStarch to patients with severe liver disease or severe bleeding disorders. With the administration of certain hydroxyethyl starch solutions, disturbances of blood coagulation can occur depending on the dosage.4,55.2 Monitoring: Laboratory Tests
Clinical evaluation and periodic laboratory determinations are necessary to monitor fluid balance, electrolyte concentrations, kidney function, acid-base balance, and coagulation parameters during prolonged parenteral therapy, or whenever the patient’s condition warrants such evaluation.
5.3 Interference with Laboratory Tests
In humans, elevated serum amylase levels may be observed temporarily following administration of the product and can interfere with the diagnosis of pancreatitis.
At high dosages the dilutional effects may result in decreased levels of coagulation factors and other plasma proteins and a decrease in hematocrit.4,5 -
6. ADVERSE REACTIONS
6.1 Overall Adverse Reaction Profile
Products containing hydroxyethyl starch may lead to anaphylactoid reactions (hypersensitivity, mild influenza-like symptoms, bradycardia, tachycardia, bronchospasm, non-cardiac pulmonary edema). In the event of an intolerance reaction, the infusion should be discontinued immediately and the appropriate emergency medical treatment initiated. [see General Warnings and Precautions (5.1)]
Skin and subcutaneous tissue disorders in humans; prolonged administration of high dosages of hydroxyethyl starch may cause pruritus (itching) which is an undesirable effect observed with all hydroxyethyl starches.
Investigations; at high doses, the dilutional effects may result in decreased levels of coagulation factors and other plasma proteins, and a decrease in hematocrit. [see Interference with Laboratory Tests (5.3)] -
7. DRUG INTERACTIONS
No interactions with other drugs or nutritional products are known. The safety and compatibility of other additives have not been established.4,5 [see Directions for Use of VetStarch (2.2)]
-
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
The type of hydroxyethyl starch present in VetStarch had no teratogenic properties in rats or rabbits. At 5 g/kg of body weight per day, administered as a bolus injection, fetal retardations and embryolethal effects were observed in rats and rabbits, respectively.
In rats, a bolus injection of this dose during pregnancy and lactation reduced body weight of offspring and induced developmental delays. All adverse effects were seen exclusively at maternal toxic doses due to fluid overload. [see Toxicology (12.2.1)]
Fertility studies on directly exposed animals have not been conducted. -
9. OVERDOSAGE
As with all plasma volume substitutes, overdosage can lead to overloading of the circulatory system (e.g., pulmonary edema). In this case, the infusion should be stopped immediately and, if necessary, a diuretic should be administered. [see General Warnings and Precautions (5.1)]
-
10. DESCRIPTION
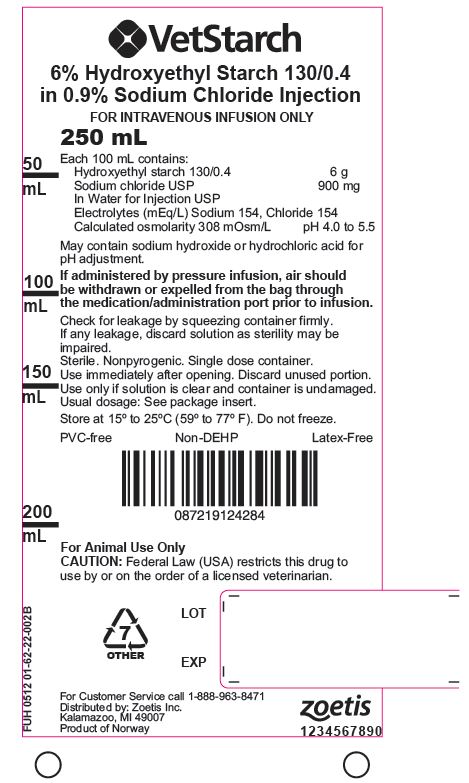
VetStarch™ (6% hydroxyethyl starch 130/0.4 in 0.9% sodium chloride injection) is a clear to slightly opalescent, colorless to slightly yellow, sterile, non-pyrogenic, isotonic solution for intravenous administration using sterile equipment.
Each 100 mL of the solution contains:
Hydroxyethyl Starch 130/0.4 6 g Sodium Chloride USP in Water for Injection USP 900 mg pH adjusted with Sodium Hydroxide USP or Hydrochloric Acid USP Electrolytes (mEq/L): Sodium 154, Chloride 154, pH 4 to 5.5. Calculated osmolarity 308 mOsmol/L. The hydroxyethyl starch contained in VetStarch is a synthetic colloid for use in plasma volume replacement. The chemical name of hydroxyethyl starch is poly(O-2-hydroxyethyl) starch. The structural formula of hydroxyethyl starch is:
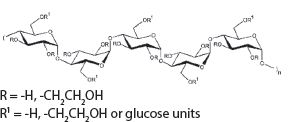
structure of Vetstarch
VetStarch is packaged in 250 mL and 500 mL flexible plastic containers made from coextruded polyolefin and is free of PVC, plasticizers, adhesives or latex (Non-DEHP, Latex-free). The container offers an air-closed system and can be used with nonvented IV sets which prevent external air contamination. The container is collapsible and can be used in emergency cases for pressure infusion.
-
11. CLINICAL PHARMACOLOGY
11.1 Mechanism of Action
VetStarch contains hydroxyethyl starch in a colloidal solution which expands plasma volume when administered intravenously. This effect depends on the mean molecular weight (130,000 daltons; range 110,000 – 150,000 daltons), the molar substitution by hydroxyethyl groups (0.4; range 0.38 – 0.45) on glucose units of the starch, the pattern of hydroxyethyl substitution (C2/C6 ratio) of approximately 9:1, and the concentration (6%), as well as the dosage and infusion rate.
Hydroxyethyl starch is a derivative of thin boiling waxy corn starch, which mainly consists of a glucose polymer (amylopectin) predominately composed of α-1-4-connected glucose units with several α-1-6-branches. Substitution of hydroxyethyl groups on the glucose units of the polymer reduces the normal degradation of amylopectin by α-amylase in the body. The low molar substitution (0.4) is the main pharmacological determinant for the beneficial effects of VetStarch on pharmacokinetics, intravascular volume and hemodilution.6 To describe the molecular weight and molar substitution characteristics of the hydroxyethyl starch in VetStarch, the compound is designated as hydroxyethyl starch 130/0.4. -
12. NONCLINICAL TOXICOLOGY
12.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals, to evaluate the carcinogenic potential of VetStarch, have not been performed. No mutagenic effects were observed with hydroxyethyl starch 130/0.4 10% solution in the following tests on mutagenic activity: Salmonella typhimurium reverse mutation assay (in vitro), mammalian cells in the in vitro gene mutation assay, assessment of the clastogenic activity in cultured human peripheral lymphocytes (in vitro), bone marrow cytogenetic test in Sprague-Dawley rats. Fertility studies on directly exposed animals have not been performed.
12.2.1 Toxicology
Three-month repeat infusion toxicology studies were conducted in rats and dogs in which three groups of animals were administered daily intravenous infusion over three hours. Dosing volumes of either 60 or 90 mL/kg body weight of hydroxyethyl starch 130/0.4 (10% solution), or 90 mL/kg 0.9% sodium chloride injection, were studied. Observed toxicity following repeat infusion of hydroxyethyl starch is consistent with the oncotic properties of the solution resulting in hypervolemia in the animals. There were no apparent gender-related effects on toxicity following repeat administration of hydroxyethyl starch 130/0.4 in rats or dogs.
In reproduction studies in rats and rabbits, hydroxyethyl starch 130/0.4 (10% solution) had no teratogenic properties. Embryolethal effects were observed in rabbits at 5 g/kg body weight/day. In rats, bolus injection of this dose during pregnancy and lactation reduced body weight of offspring and induced developmental delays. Signs of fluid overload were seen in the dams. Hydroxyethyl starch 130/0.4 (10% solution) was observed to have no effect in studies assessing skin sensitization, antigenicity, and blood compatibility.
12.2.2 Pharmacology
The pharmacodynamic effect of VetStarch™ was examined in a hemorrhagic shock model in conscious rats, and a hemodilution model in dogs. In both studies, the control group received pentastarch (6% hydroxyethyl starch 200/0.5).
VetStarch was as effective as pentastarch in maintaining cardiopulmonary function during isovolemic hemodilution in beagle dogs. In the three-hour follow-up period, no additional administration of colloid was necessary.
There were no differences in long-term survival of rats after a single administration of VetStarch and pentastarch solutions following induced hemorrhagic shock (67% and 50% blood loss). In the 67% induced bleeding group receiving VetStarch (N=6), the survival rate was 83% which is within the normal range for this type of experiment. In the corresponding pentastarch group, survival was 100%. Infusion of Ringer’s lactate resulted in a 50% survival rate after a 50% blood loss and a 0% survival after a 67% blood loss. After multiple intravenous infusions of 0.7 g per kg body weight per day of 10% hydroxyethyl starch 130/0.4, or 10% hydroxyethyl starch 200/0.5 solution, during 18 consecutive days, the plasma hydroxyethyl starch concentration in rats treated with hydroxyethyl starch 130/0.4 was lower compared to rats treated with hydroxyethyl starch 200/0.5. Hydroxyethyl starch 130/0.4 was eliminated faster than hydroxyethyl starch 200/0.5. In both groups, clear signs of hydroxyethyl starch tissue storage were detected in lymph nodes and spleen. Numerous empty vacuoles in macrophages were observed. Only minimal cellular vacuolization was found in the liver and kidney. Histochemical differences between the groups were not observed.A study with 10% radiolabeled 14C-hydroxyethyl starch 130/0.4 and 10% 14C-hydroxyethyl starch 200/0.5 solutions was carried out.7 In animals treated with hydroxyethyl starch 130/0.4, radioactivity decreased from 4.3% of the total administered dose (2.6 g hydroxyethyl starch 130/0.4 per animal) on day 3 to 0.65% on day 52. In animals treated with hydroxyethyl starch 200/0.5, the 14C activity decreased from 7.7% of the total administered dose (2.7 g hydroxyethyl starch 200/0.5 per animal) on day 3 to 2.45% on day 52. These results confirm the faster elimination and lower persistence of hydroxyethyl starch 130/0.4 in tissue.
-
13. REFERENCES
1. Silverstein D, Hopper K. Small Animal Critical Care Medicine. (2009).
2. Neff T., Doelberg M., Jungheinrich C., Sauerland A., Spahn D., Stocker R., “Repetitive Large-Dose Infusion of the Novel Hydroxyethyl Starch 130/0.4 in Patients with Severe Head Injury.” Anesth Analg 96 (2003): 1453-9
3. DiBartola, S. Fluid, Electrolyte, & Acid-Base Disorders in Small Animal Practice. (2011).
4. Smiley, et al. “The Use of Hetastarch as Adjunct Therapy in 36 dogs with Hypoalbuminemia: a Phase Two Clinical Trial.”J Vet Intern Med, 8 (1994): 195-200.
5. Smart, et al. “The Effect of Hetastarch (670/0.75) In Vivo on Platelet Closure Time in the Dog.” J Vet Emerg Crit Care, 19(5) (2009): 444-449.
6. Jungheinrich, C., Neff, T. “Pharmacokinetics of hydroxyethyl starch.” Clin Pharmacokinetik 44 (7) (2005): 681-699.
7. Leuschner, J., Opitz, J., Winkler, A., Scharpf, R., Bepperling, F. “Tissue storage of 14C-labeled hydroxyethyl starch (HES) 130/0.4 and HES 200/0.5 after repeated intravenous administration to rats.” Drugs R D, 4 (6) (2003): 331-8. -
14. HOW SUPPLIED / STORAGE AND HANDLING
VetStarch™ (6% hydroxyethyl starch 130/0.4 in 0.9% sodium chloride injection) for intravenous infusion is supplied in the following primary container and carton sizes:
Polyolefin bag with overwrap: 250 mL and 500 mL
Carton of 20 x 500 mL
Carton of 30 x 250 mL
Store at 15° to 25°C (59° to 77°F), Do not freezeFor Customer Service call 1-888-963-8471
Distributed by:
Zoetis Inc.
Kalamazoo, MI 49007
Product of Norway
- PRINCIPAL DISPLAY PANEL 250 ml
- PRINCIPAL DISPLAY PANEL 500 ML
-
INGREDIENTS AND APPEARANCE
VETSTARCH
hydroxyethyl starch 130/0.4 substitution injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-4950 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROXYETHYL STARCH 130/0.4 (UNII: 1GVO236S58) (HYDROXYETHYL STARCH 130/0.4 - UNII:1GVO236S58) HYDROXYETHYL STARCH 130/0.4 6000 mg in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 900 mg in 100 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-4950-2 250 mL in 1 BAG 2 NDC:54771-4950-5 500 mL in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 02/10/2012 Labeler - Zoetis Inc. (828851555) Establishment Name Address ID/FEI Business Operations Fresenius Kabi Austria 300206604 API MANUFACTURE Establishment Name Address ID/FEI Business Operations Fresenius Kabi Norge As 731170932 MANUFACTURE