Label: LOPERAMIDE HCL AND SIMETHICONE tablet
- NDC Code(s): 51660-069-06, 51660-069-12, 51660-069-18, 51660-069-24
- Packager: Ohm Laboratories Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 30, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
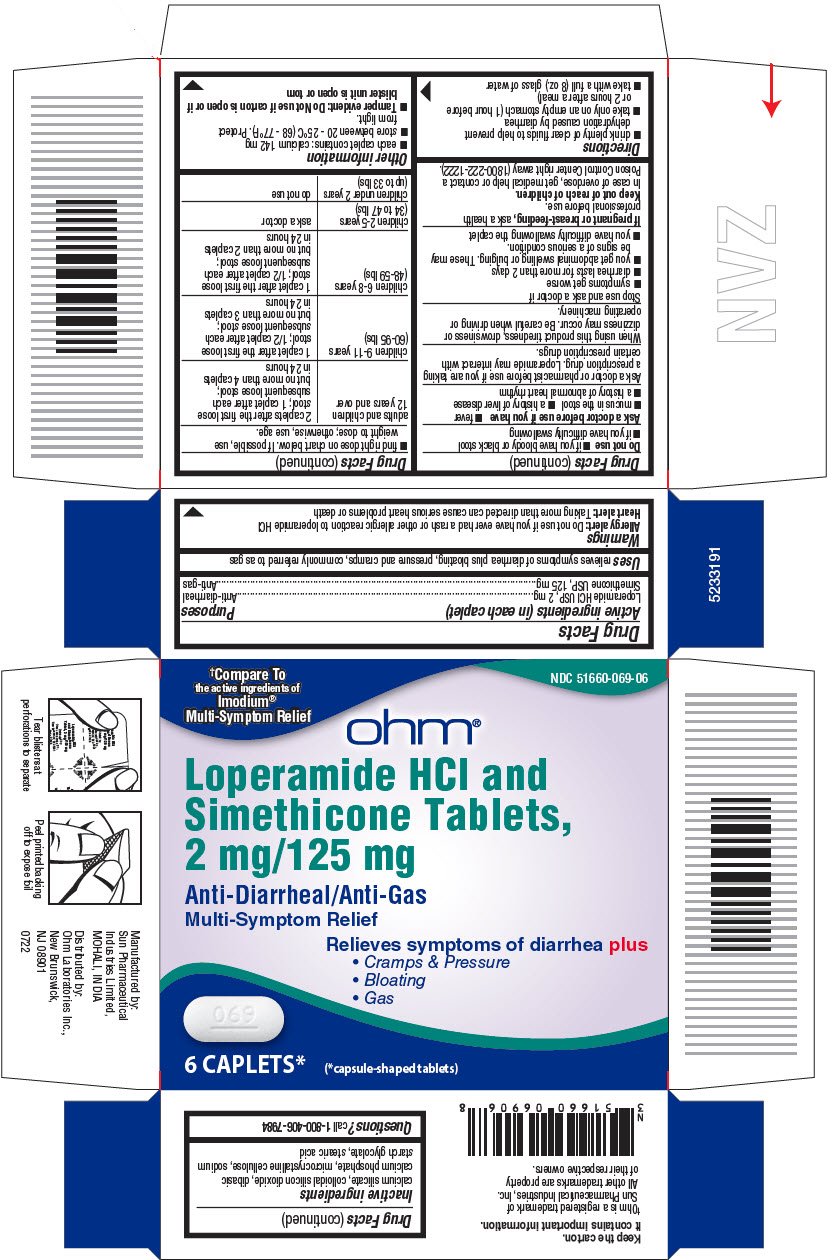
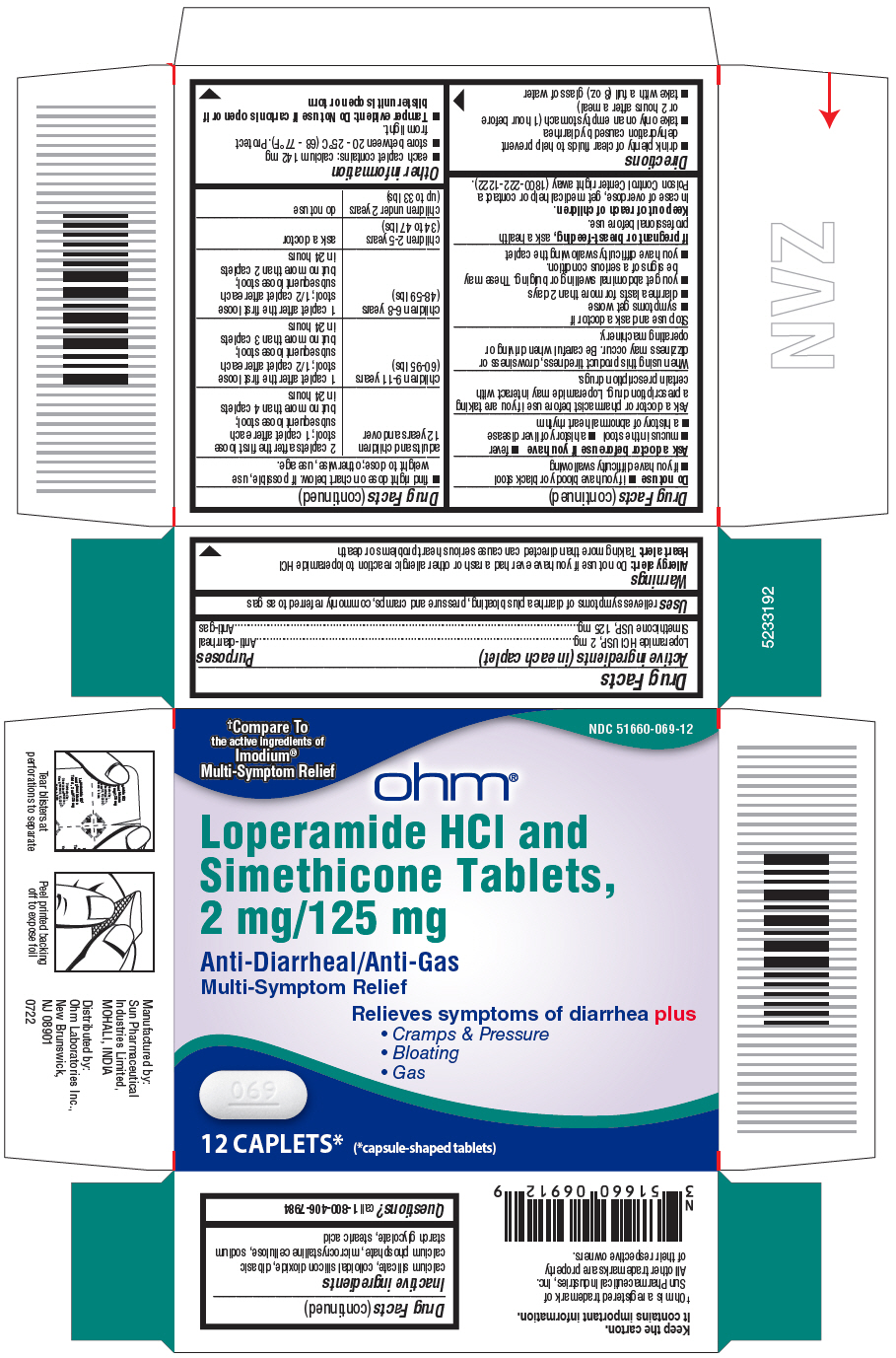
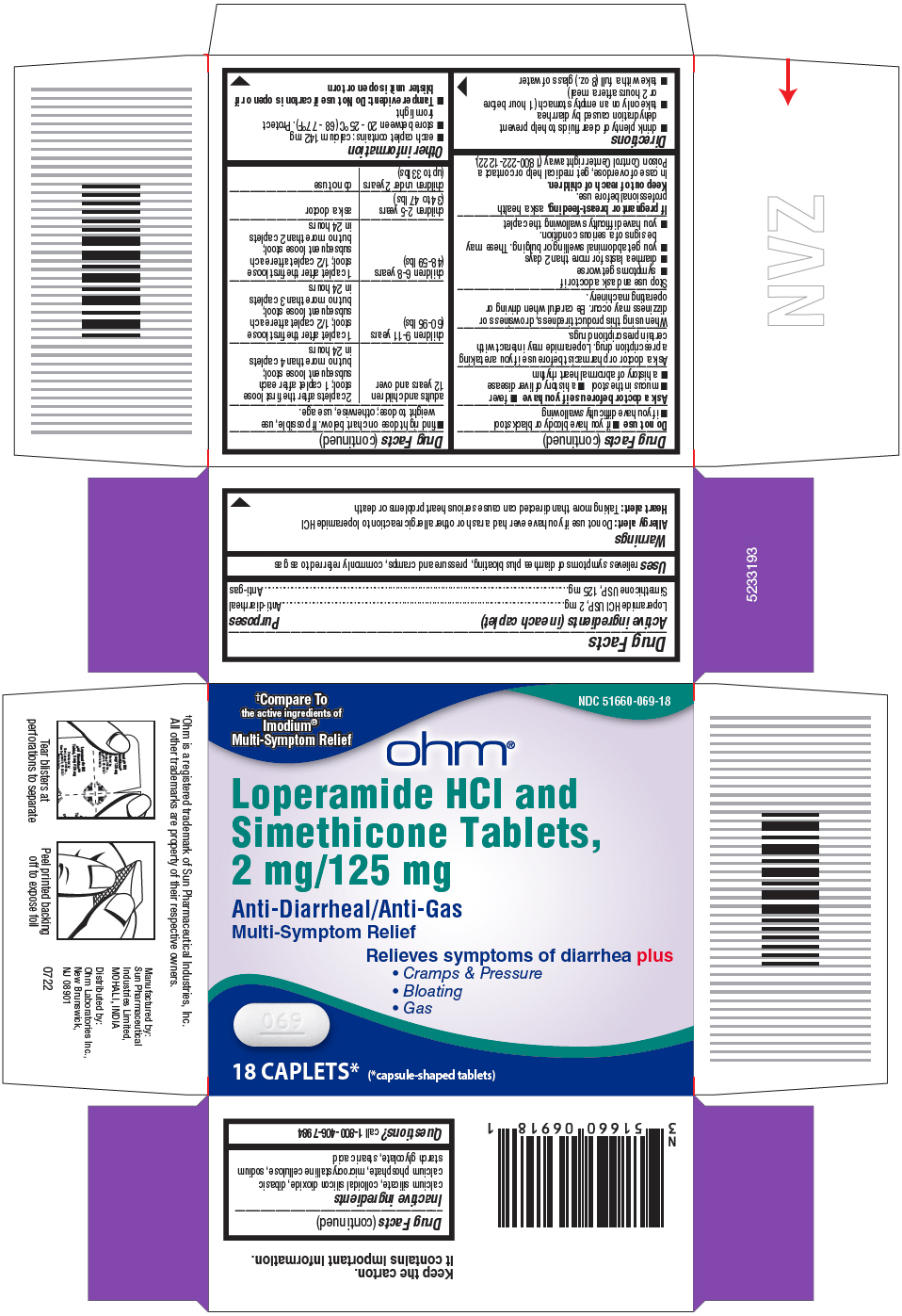
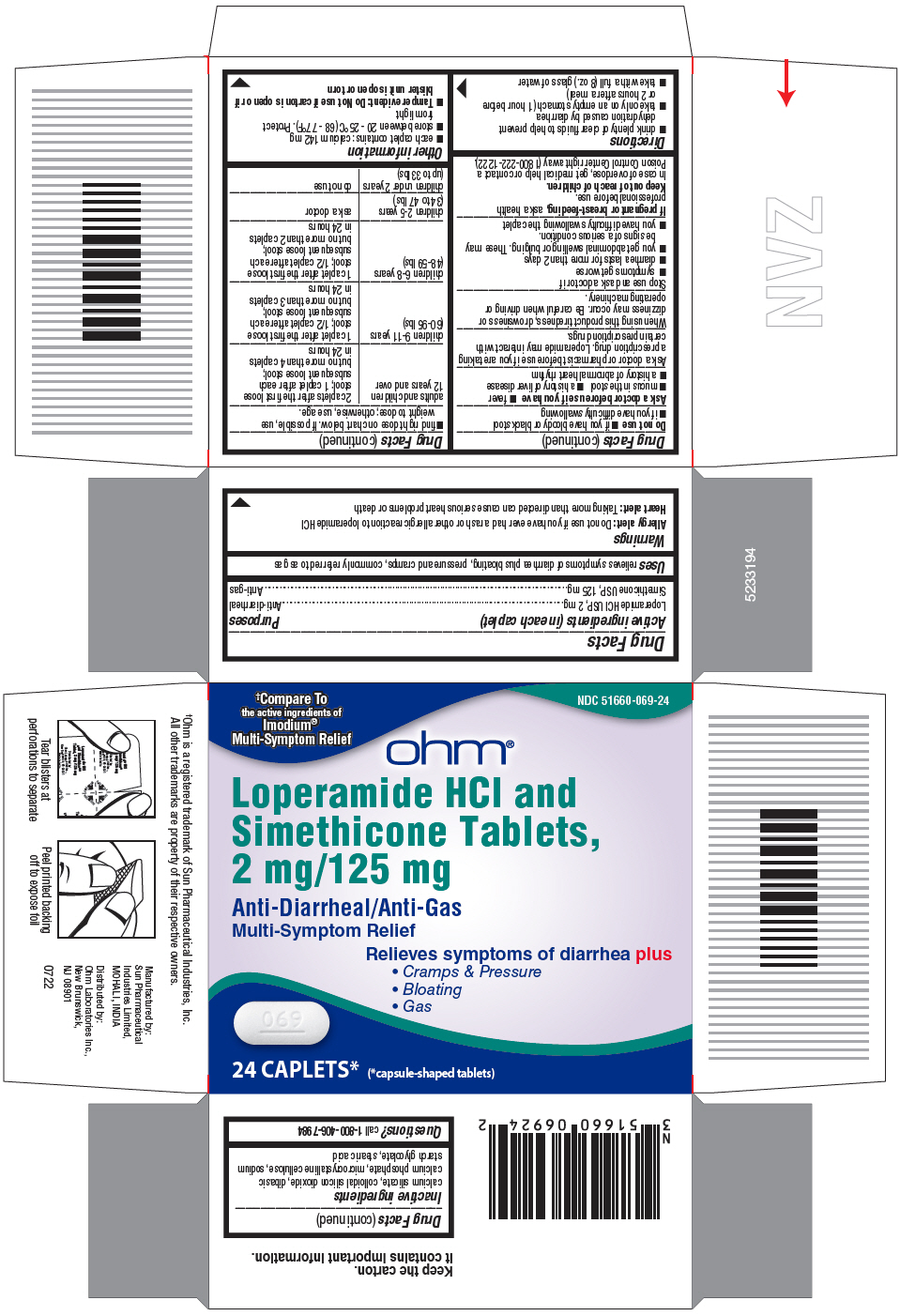
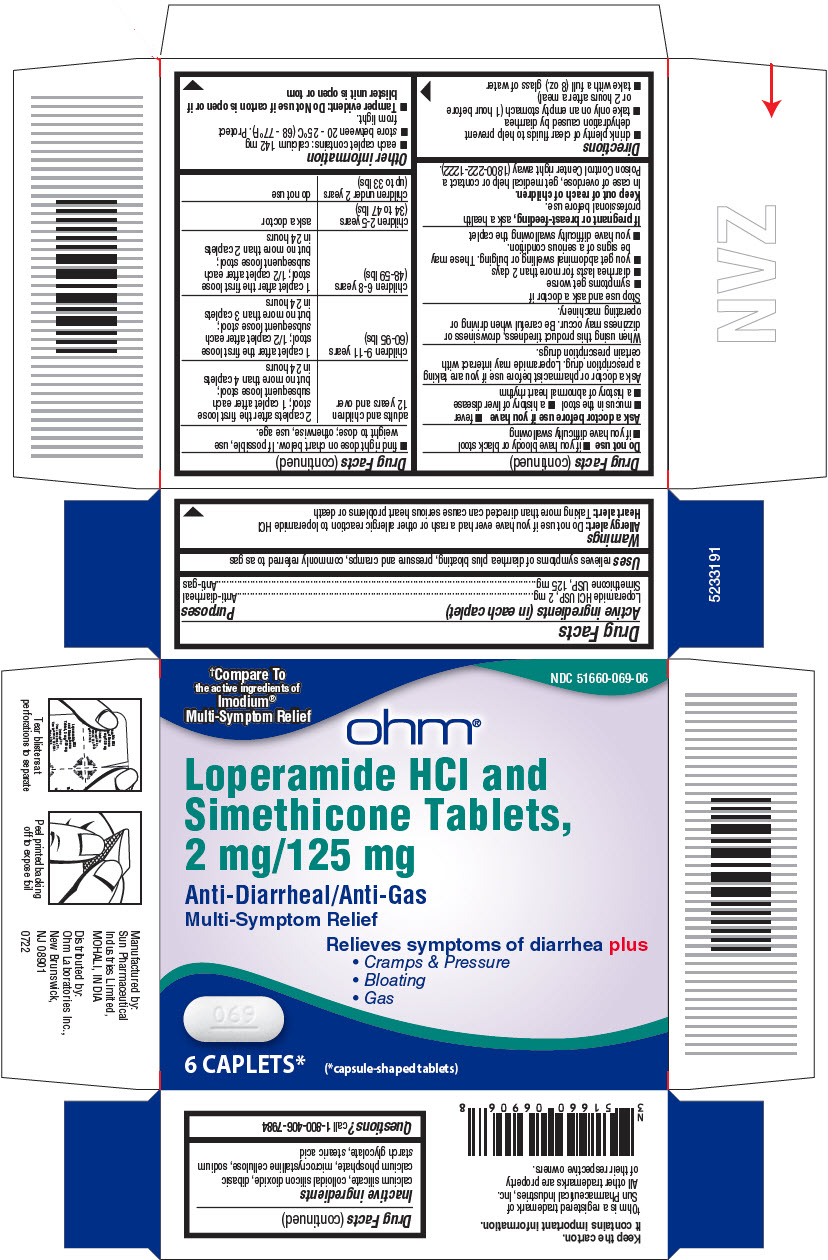
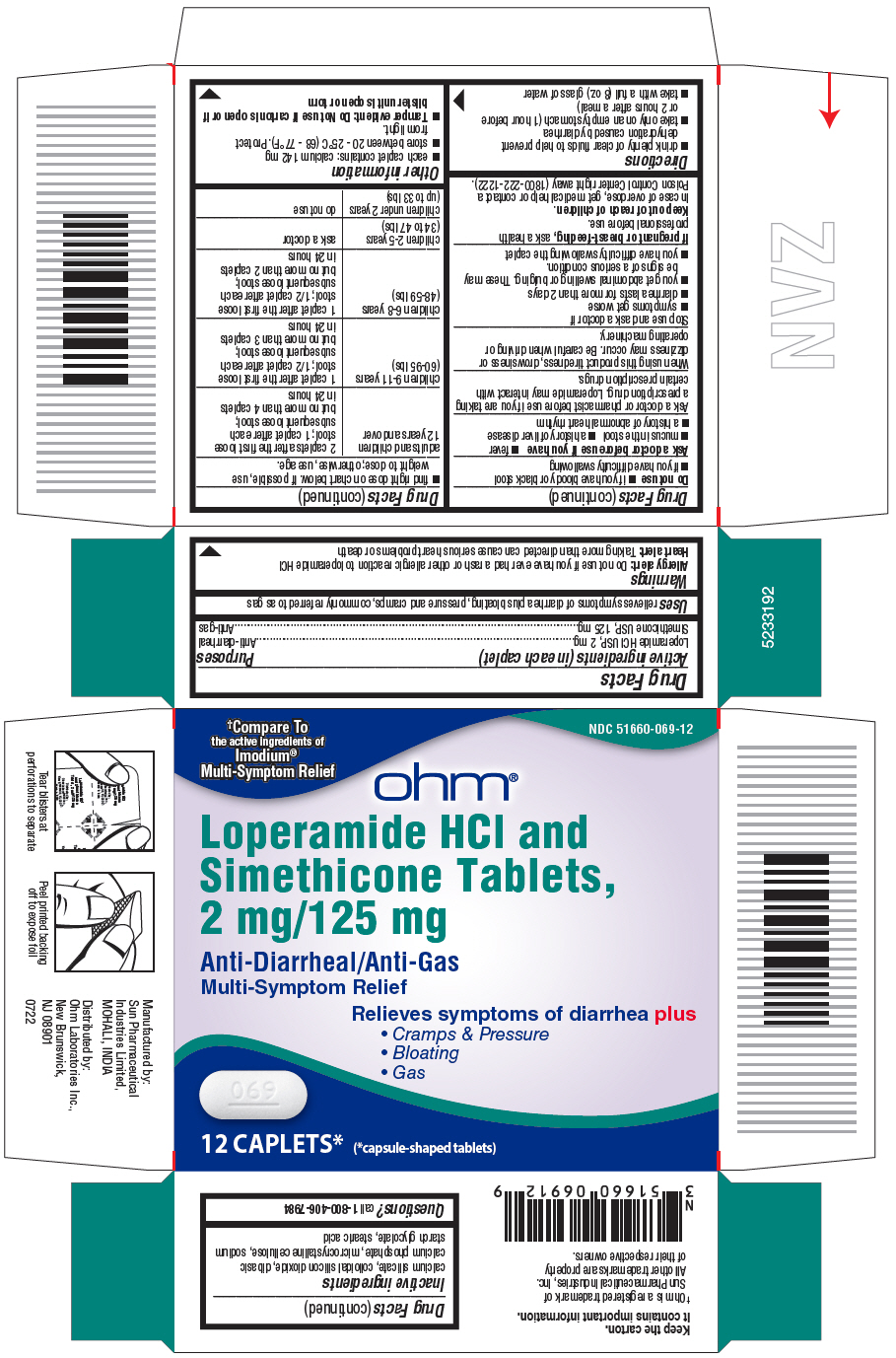
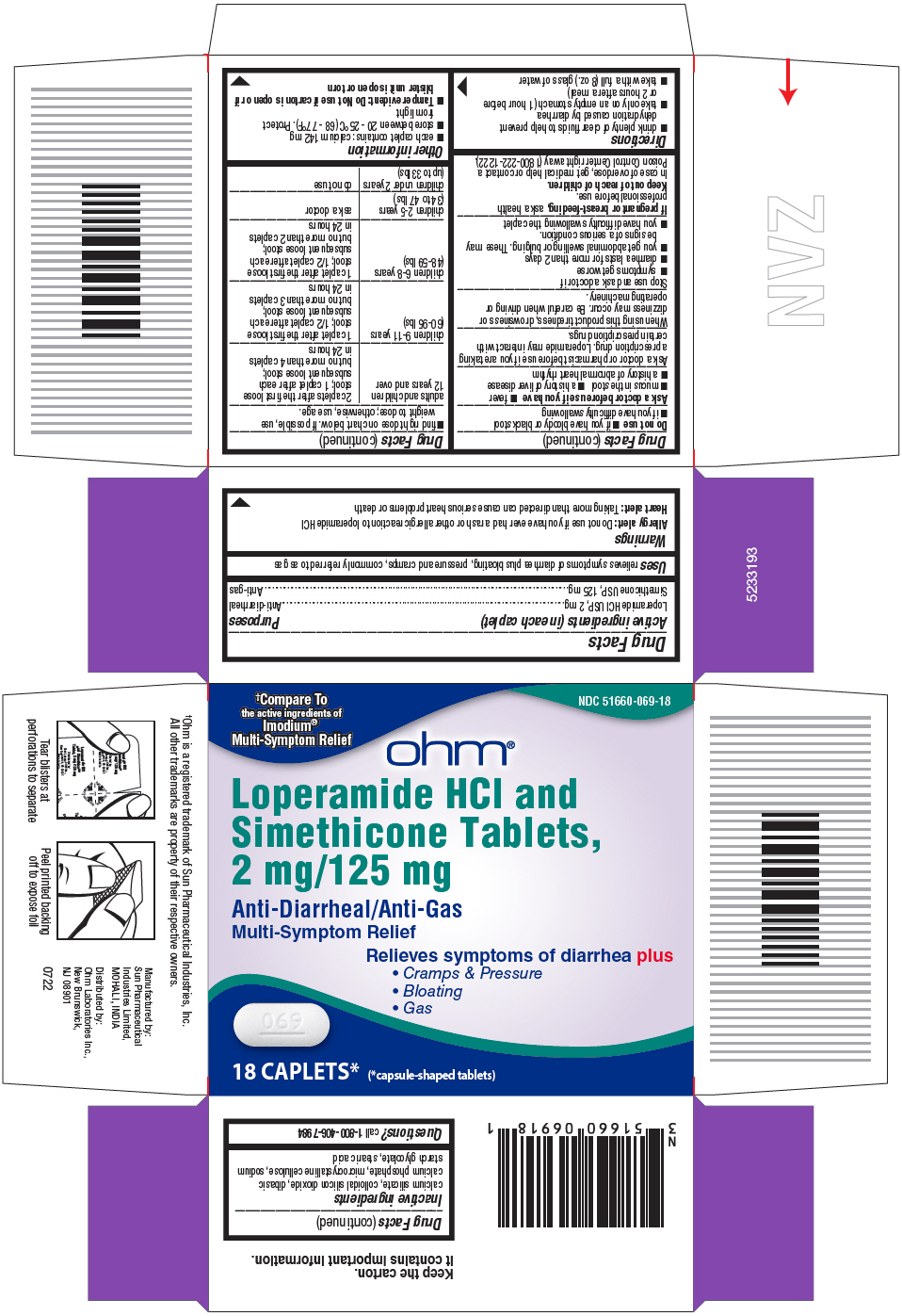
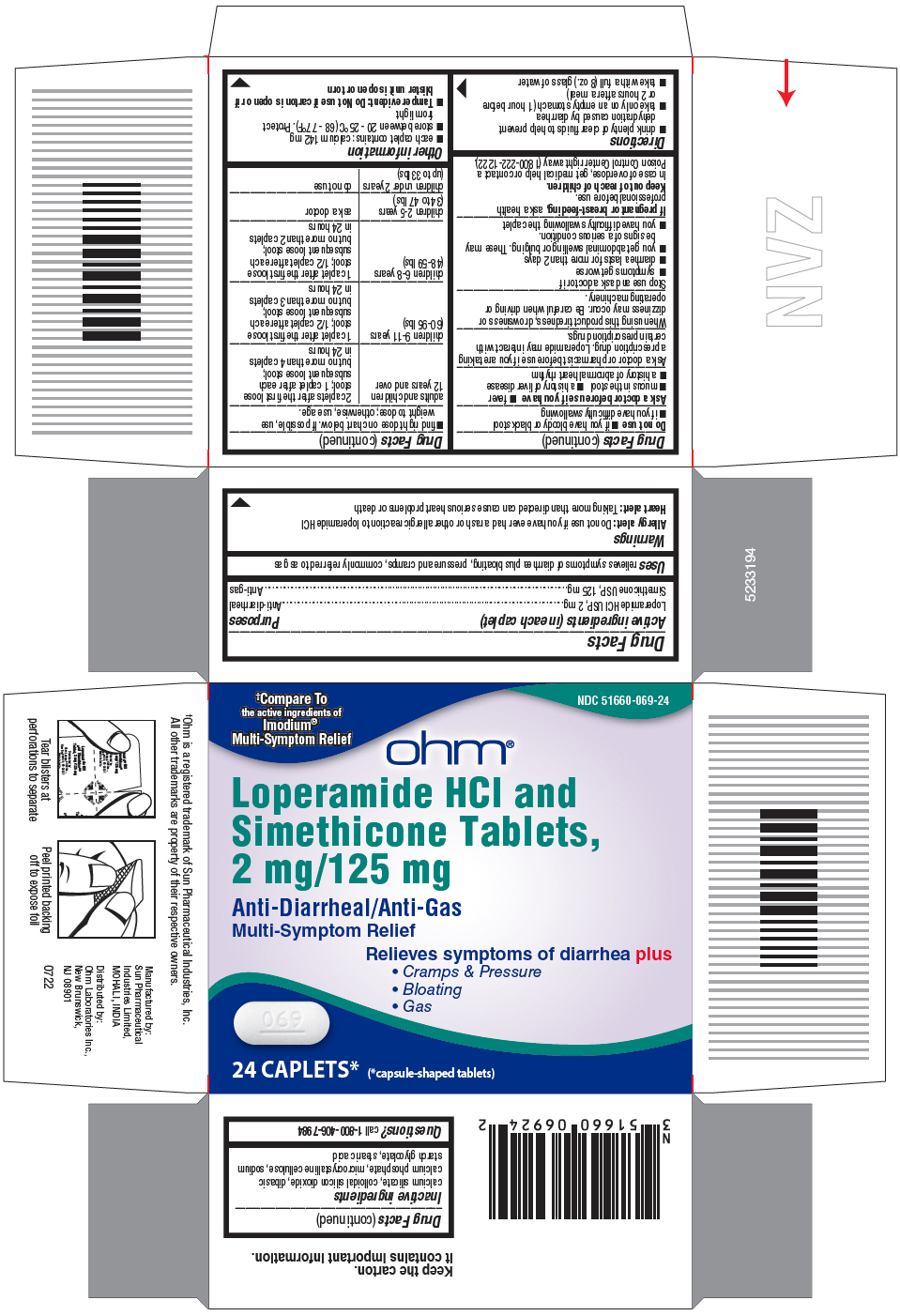
- Active ingredients (in each caplet)
- Purposes
- Uses
-
Warnings
Allergy alert:
Do not use if you have ever had a rash or other allergic reaction to loperamide HCl
Heart Alert: Taking more than directed can cause serious heart problems or death
Ask a doctor before use if you have
- •
- fever
- •
- mucus in the stool
- •
- a history of liver disease
- •
- a history of abnormal heart rhythm
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Loperamide may interact with certain prescription drugs.
When using this product
tiredness, drowsiness or dizziness may occur. Be careful when driving or operating machinery.
-
Directions
- •
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- •
- take only on an empty stomach (1 hour before or 2 hours after a meal)
- •
- take with a full (8 oz.) glass of water
- •
- find right dose on chart below. If possible, use weight to dose; otherwise, use age.
adults and children 12 years and over
2 caplets after the first loose
stool; 1 caplet after each
subsequent loose stool;
but no more than 4 caplets in 24 hours
children 9-11 years (60-95 lbs)
1 caplet after the first loose
stool; 1/2 caplet after each
subsequent loose stool;
but no more than 3 caplets in 24 hours
children 6-8 years (48-59 lbs)
1 caplet after the first loose
stool; 1/2 caplet after each
subsequent loose stool;
but no more than 2 caplets in 24 hours
children 2-5 years (34 to 47 lbs)
ask a doctor
children under 2 years (upto 33 lbs)
do not use
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 6 Capsule Blister Pack Carton
- PRINCIPAL DISPLAY PANEL - 12 Caplet Blister Pack Carton
- PRINCIPAL DISPLAY PANEL - 18 Caplet Blister Pack Carton
- PRINCIPAL DISPLAY PANEL - 24 Caplet Blister Pack Carton
-
INGREDIENTS AND APPEARANCE
LOPERAMIDE HCL AND SIMETHICONE
loperamide hcl and simethicone tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51660-069 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LOPERAMIDE HYDROCHLORIDE (UNII: 77TI35393C) (LOPERAMIDE - UNII:6X9OC3H4II) LOPERAMIDE HYDROCHLORIDE 2 mg DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 125 mg Inactive Ingredients Ingredient Name Strength CALCIUM SILICATE (UNII: S4255P4G5M) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color WHITE (white to off-white) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code NA Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51660-069-06 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 01/13/2022 2 NDC:51660-069-12 2 in 1 CARTON 01/13/2022 2 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:51660-069-18 3 in 1 CARTON 01/13/2022 3 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:51660-069-24 4 in 1 CARTON 01/13/2022 4 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077500 01/13/2022 Labeler - Ohm Laboratories Inc. (184769029) Registrant - Sun Pharmaceutical Industries, Inc. (146974886) Establishment Name Address ID/FEI Business Operations Sun Pharmaceutical Industries Limited 650456002 MANUFACTURE(51660-069)