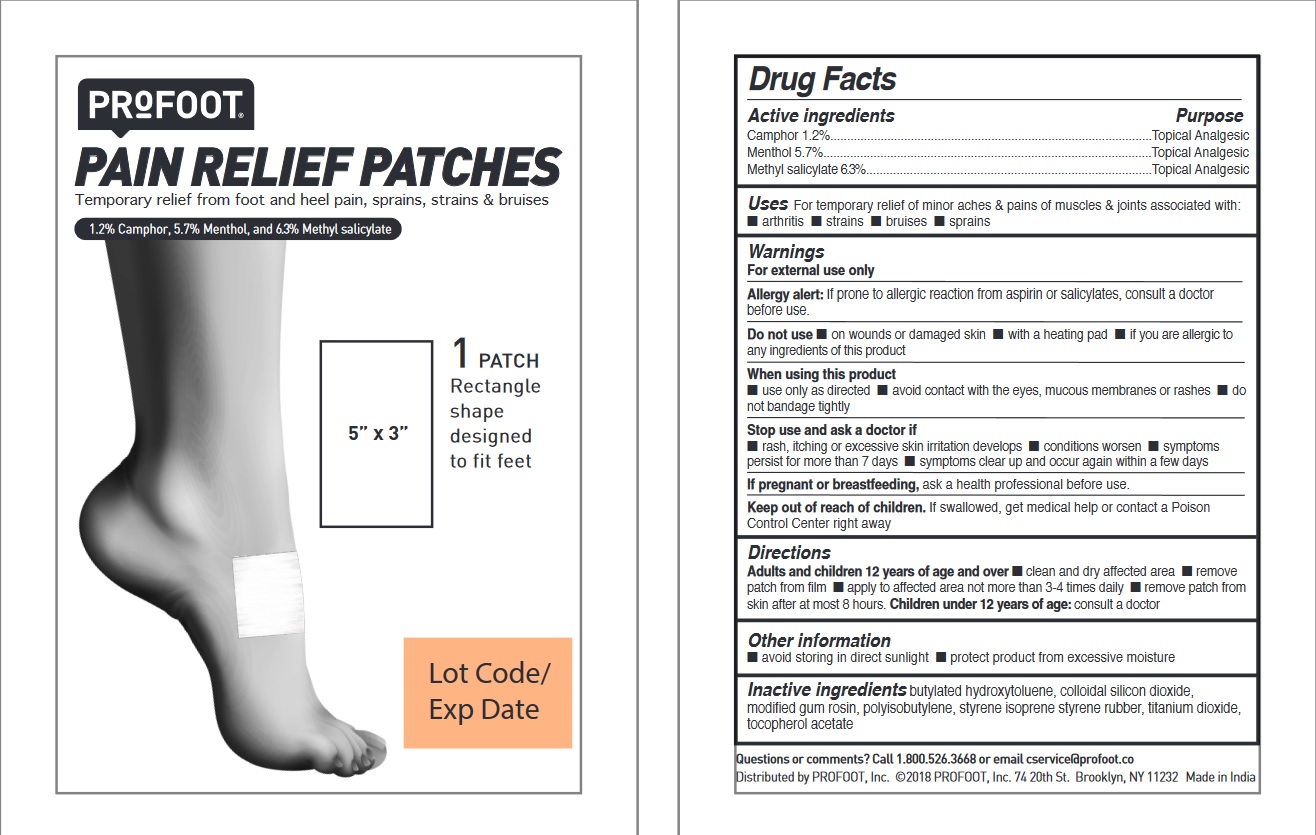
Label: PROFOOT PAIN RELIEF PATCHES- camphor, menthol, methyl salicylate kit
- NDC Code(s): 29784-121-36, 29784-122-36, 29784-601-01
- Packager: Profoot, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
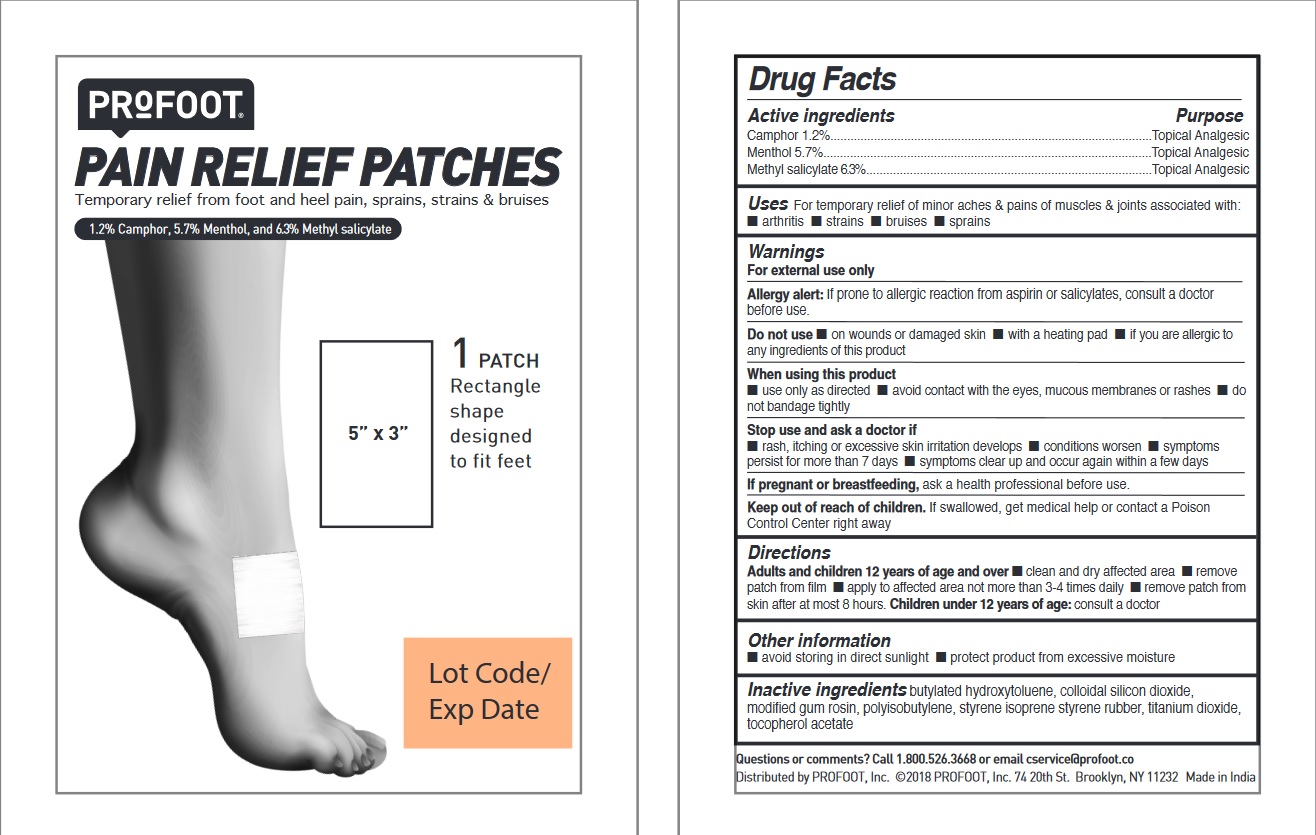
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only
Allergy alert: If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
Do not use
• on wounds or damaged skin • with a heating pad • if you are allergic to any of the ingredients of
this product
When using this product
• use only as directed • avoid contact with the eyes, mucous membranes or rashes • do not
bandage tightly
Stop use and ask a doctor if
• rash, itching or excessive skin irritation develops • condition worsens •symptoms persist
for more than 7 days • symptoms clear up and occur again within a few days
If pregnant or breastfeeding, ask a health professional before use.
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PROFOOT PAIN RELIEF PATCHES
camphor, menthol, methyl salicylate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:29784-601 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:29784-601-01 1 in 1 KIT 10/12/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PATCH 0.36 g Part 2 1 PATCH 1.3 g Part 1 of 2 PROFOOT
camphor, menthol, methyl salicylate patchProduct Information Item Code (Source) NDC:29784-121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 63 mg in 1 g CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 12 mg in 1 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 57 mg in 1 g Inactive Ingredients Ingredient Name Strength STYRENE/ISOPRENE/STYRENE BLOCK COPOLYMER (UNII: K7S96QM8DV) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) MINERAL OIL (UNII: T5L8T28FGP) HYDROGENATED C6-20 POLYOLEFIN (100 CST) (UNII: 39EYQ1W9RB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 KIT 1 NDC:29784-121-36 0.36 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/12/2023 Part 2 of 2 PROFOOT
camphor, menthol, methyl salicylate patchProduct Information Item Code (Source) NDC:29784-122 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 12 mg in 1 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 57 mg in 1 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 63 mg in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) HYDROGENATED C6-20 POLYOLEFIN (100 CST) (UNII: 39EYQ1W9RB) STYRENE/ISOPRENE/STYRENE BLOCK COPOLYMER (UNII: K7S96QM8DV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 KIT 1 NDC:29784-122-36 1.3 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/12/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/12/2023 Labeler - Profoot, Inc. (107570900)