Label: ASPER FLEX- lidocaine cream
- NDC Code(s): 71399-0049-1, 71399-0049-3, 71399-0049-5
- Packager: Akron Pharma Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- DO NOT USE
- Directions
- For external use only
- Other information
- Inactive ingredients
- Questions or comments?
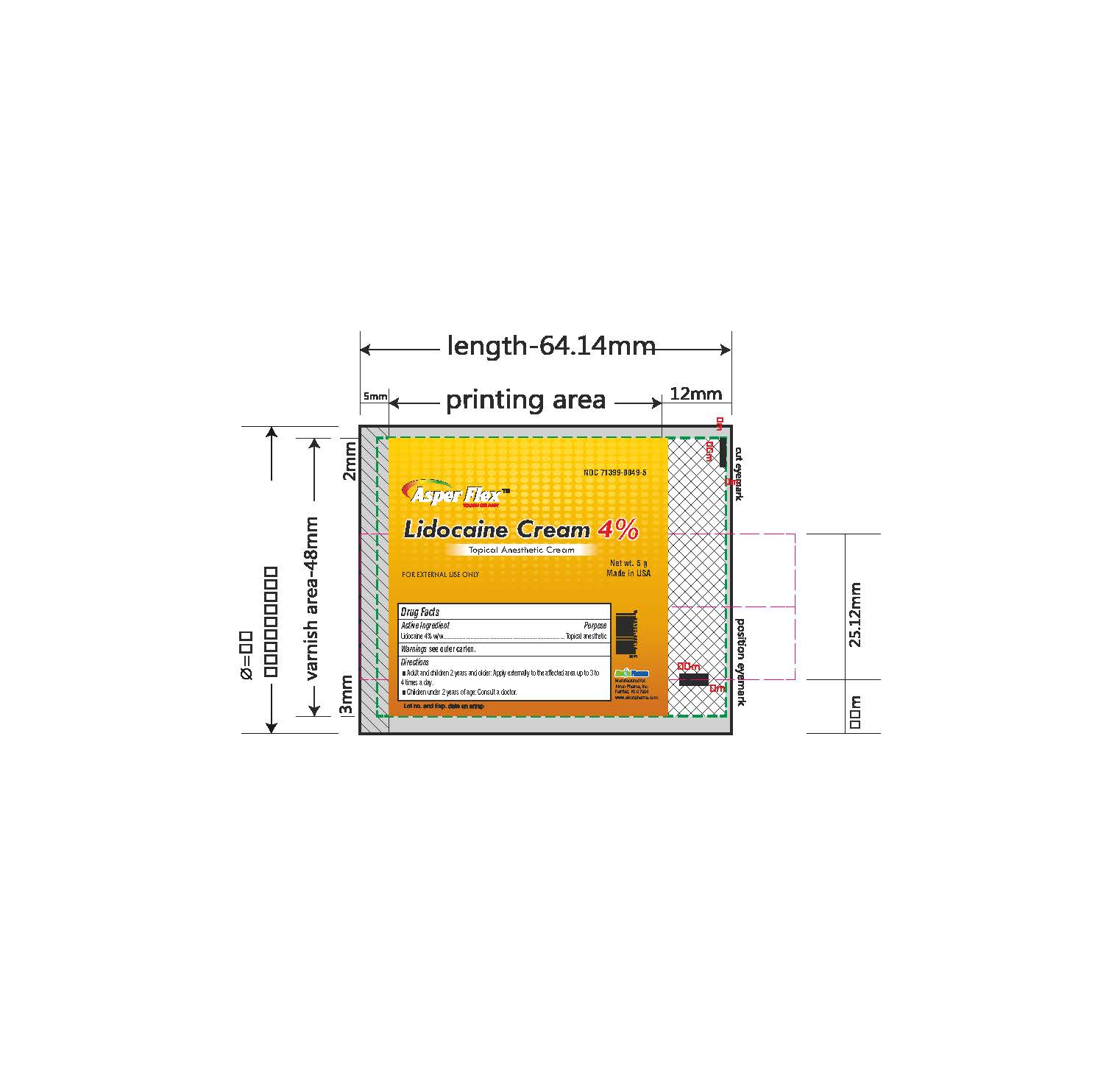
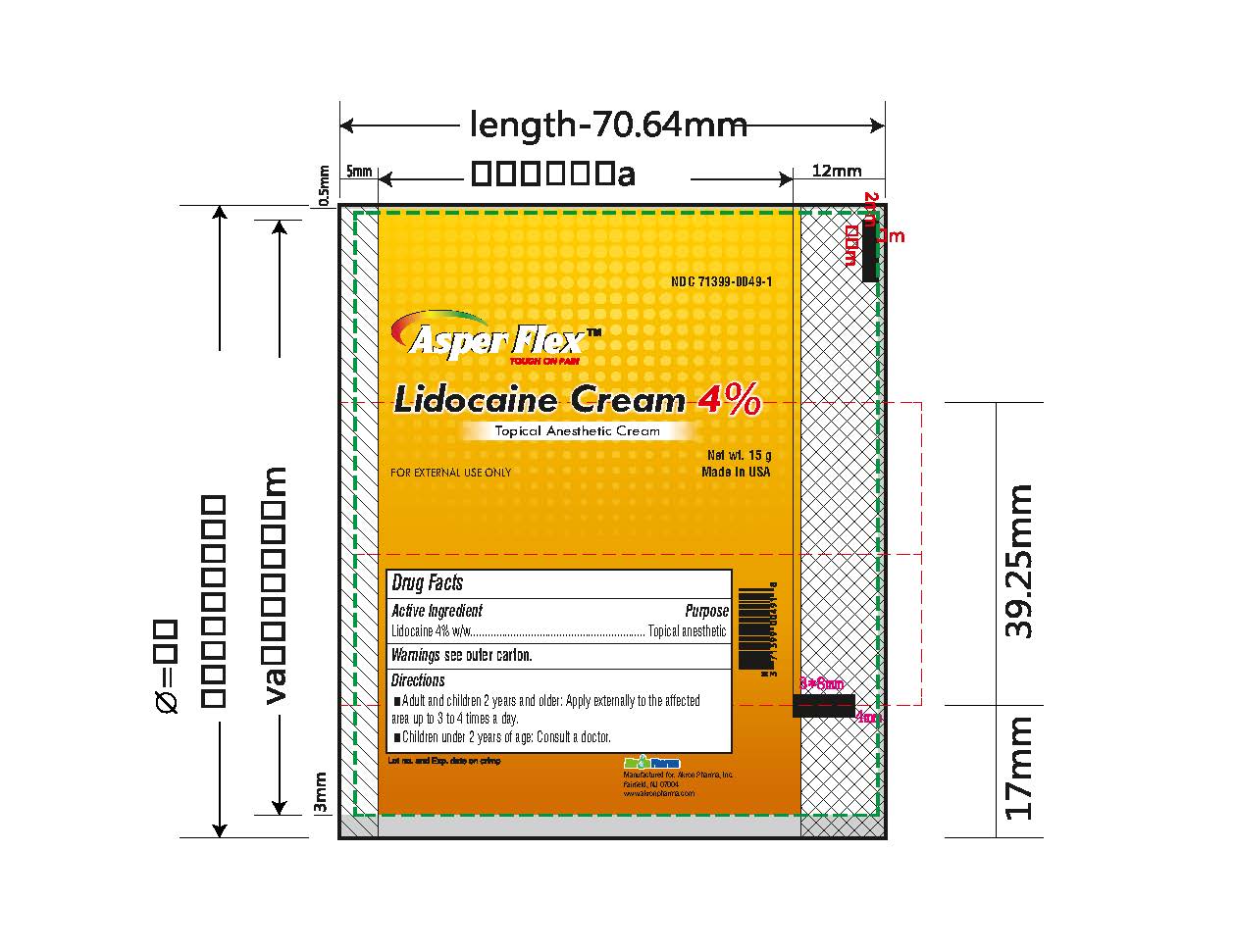
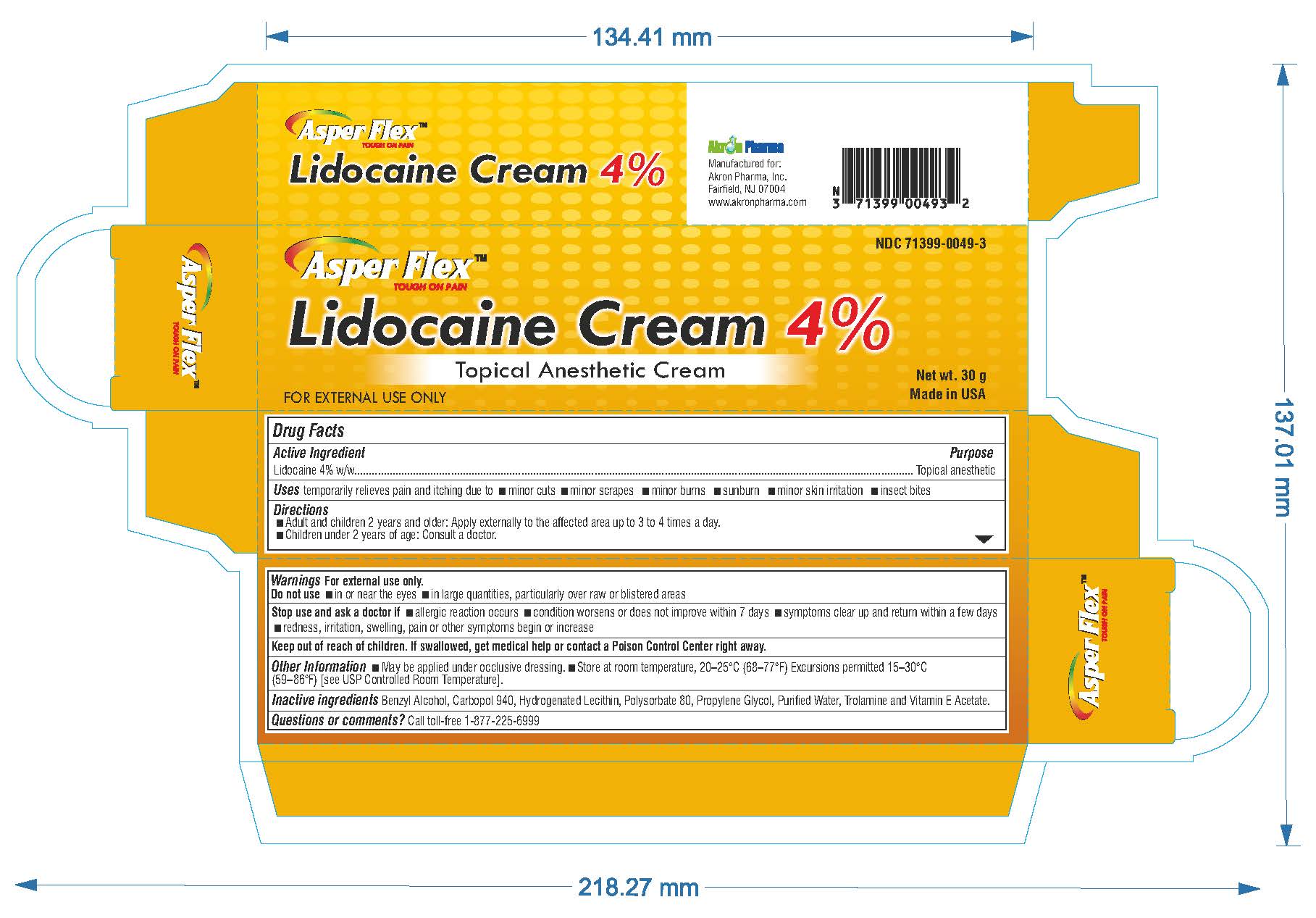
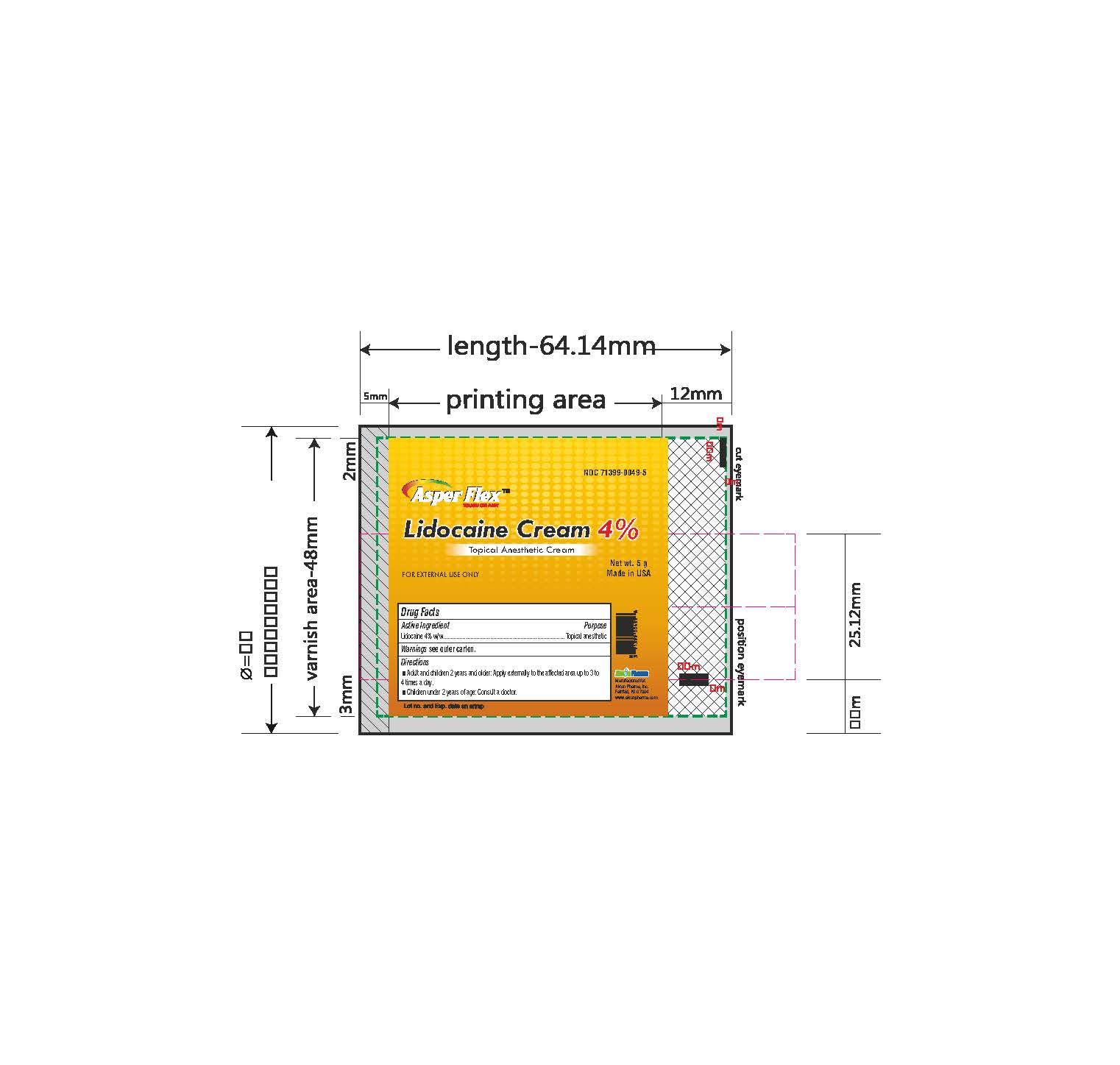
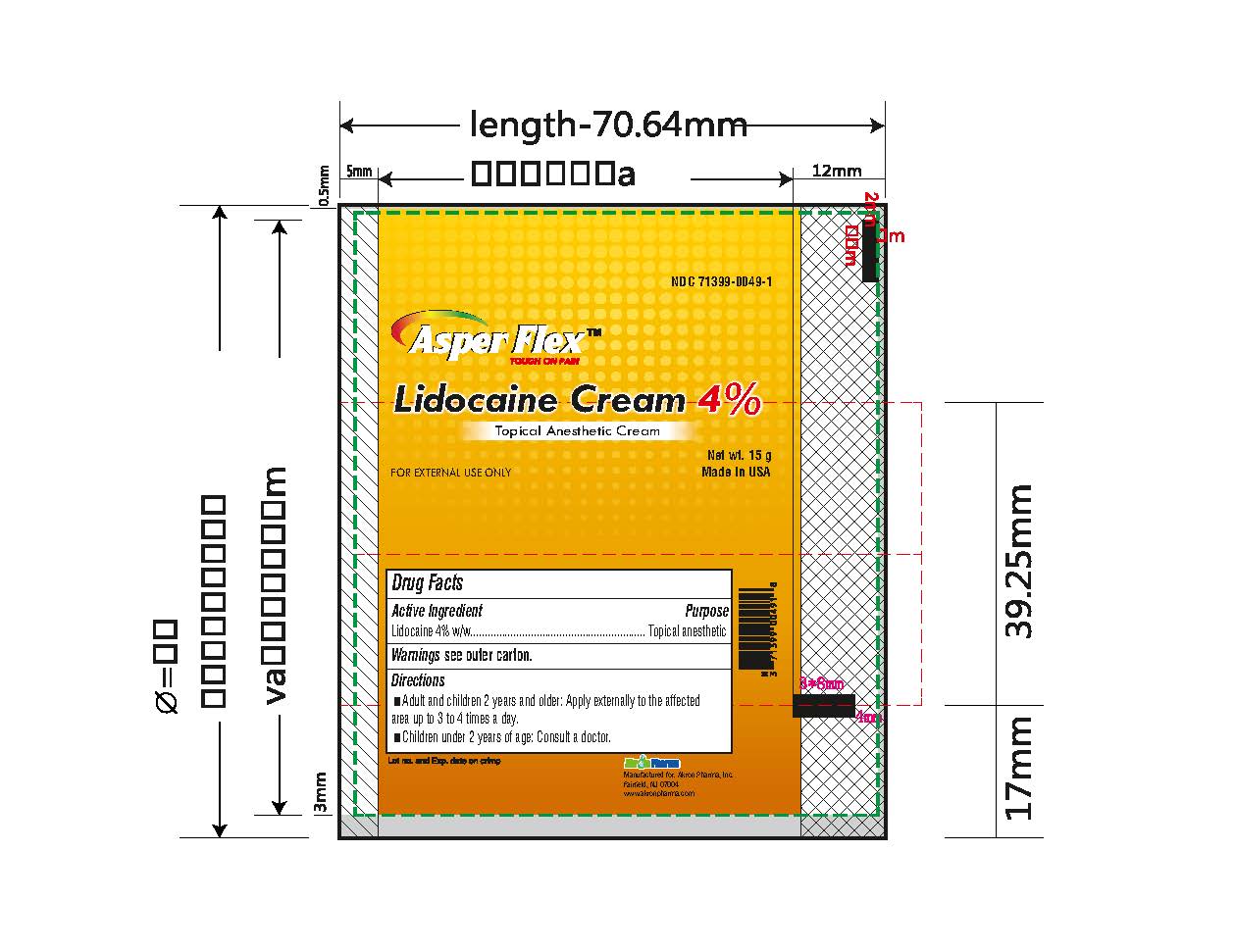
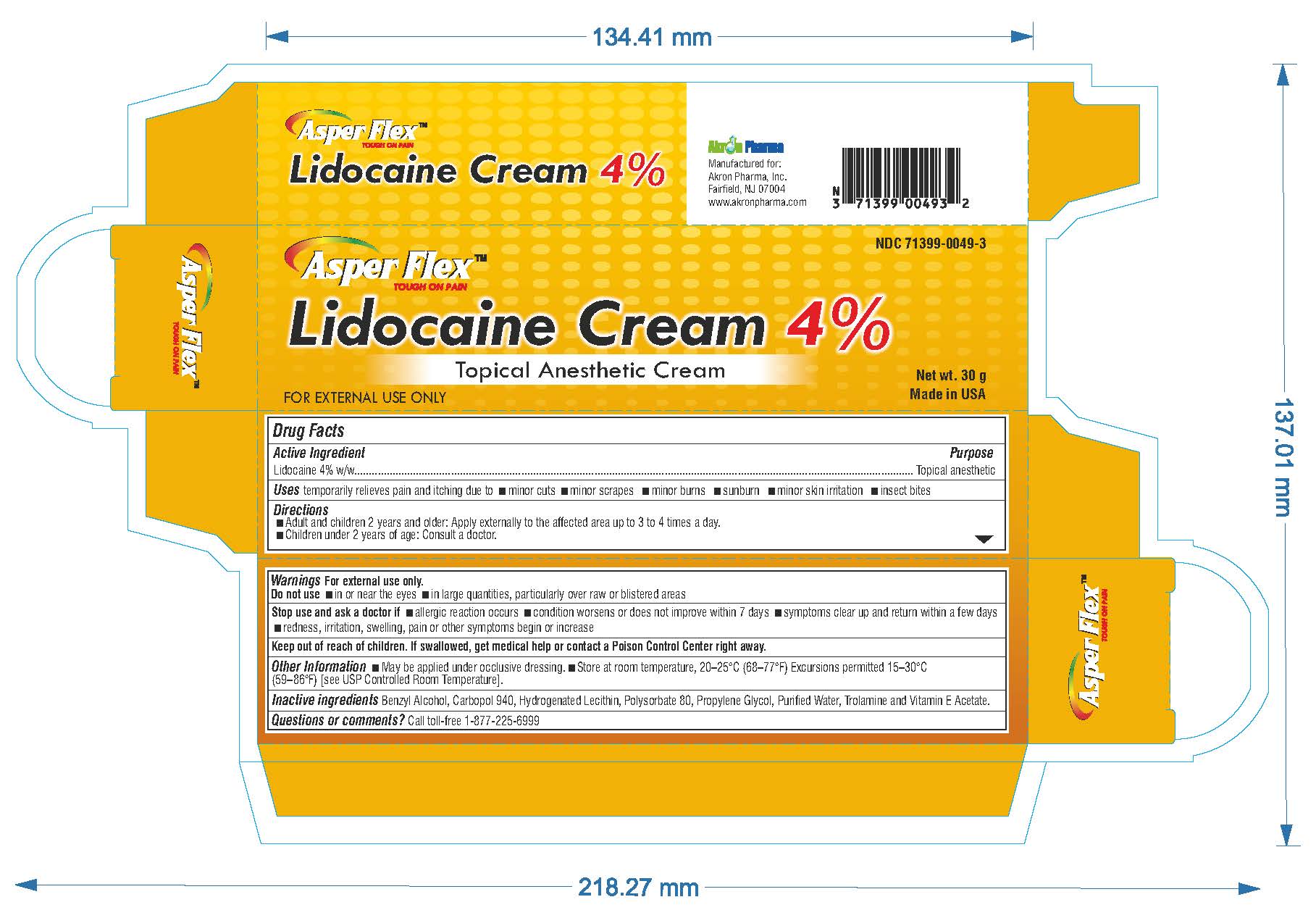
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ASPER FLEX
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71399-0049 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CARBOMER 940 (UNII: 4Q93RCW27E) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) TROLAMINE (UNII: 9O3K93S3TK) Product Characteristics Color white (clear gel) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71399-0049-5 1 in 1 CARTON 11/03/2023 1 5 g in 1 PACKAGE; Type 0: Not a Combination Product 2 NDC:71399-0049-1 1 in 1 CARTON 11/03/2023 2 15 g in 1 PACKAGE; Type 0: Not a Combination Product 3 NDC:71399-0049-3 1 in 1 CARTON 11/03/2023 3 30 g in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/03/2023 Labeler - Akron Pharma Inc. (067878881)