Label: CITRANATAL ASSURE- ascorbic acid, calcium citrate, iron, vitamin d, dl- alpha- tocopherol acetate, thiamine, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, iodine, zinc, copper, docusate sodium, doconexent and icosapent kit
- NDC Code(s): 0178-0891-30
- Packager: Mission Pharmacal Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
CitraNatal Assure ®is a prescription prenatal/postnatal multivitamin/mineral tablet with Ferr-Ease ®, a patented dual-iron delivery comprising both a quick release and slow release iron, and a capsule of an essential fatty acid. The prenatal vitamin is a white, coated, oval multivitamin/mineral tablet. The tablet is debossed "0891" on one side and is blank on the other. The essential fatty acid DHA capsule is caramel colored and contains a light yellow to orange semi-solid mixture.
Each prenatal capsule contains: Vitamin C (Ascorbic acid) 120 mg Calcium (Calcium citrate) 124 mg Iron (Carbonyl iron, ferrous gluconate) 35 mg Vitamin D 3(Cholecalciferol) 400 IU Vitamin E (dl-alpha tocopheryl acetate) 30 IU Thiamin (Vitamin B 1) 3 mg Riboflavin (Vitamin B 2) 3.4 mg Niacinamide (Vitamin B 3) 20 mg Vitamin B 6(Pyridoxine HCl) 25 mg Folic Acid 1 mg Iodine (Potassium iodide) 150 mcg Zinc (Zinc oxide) 25 mg Copper (Cupric oxide) 2 mg Docusate Sodium 50 mg Each DHA gelatin capsule contains: Docosahexaenoic Acid (DHA, 40% from 750 mg Algal Oil) 300 mg Eicosapentaenoic Acid (EPA) Not more than 0.750 mg Other ingredients in DHA gelatin capsule: Gelatin, sunflower oil, glycerin, caramel color, sunflower lecithin, rosemary extract, tocopherols, ascorbyl palmitate.
- INDICATIONS
- CONTRAINDICATIONS
- BOXED WARNING (What is this?)
-
WARNING
Ingestion of more than 3 grams of omega-3 fatty acids per day has been shown to have potential antithrombotic effects, including an increased bleeding time and INR. Administration of omega-3 fatty acids should be avoided in patients on anticoagulants and in those known to have an inherited or acquired bleeding diathesis.
- WARNING
- PRECAUTIONS
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION
- SPL UNCLASSIFIED SECTION
- STORAGE
-
SPL UNCLASSIFIED SECTION
To report a serious adverse event or obtain product information, call (210) 696-8400.
51062R1120
MISSION ®PHARMACAL
DHA capsules manufactured for:
MISSION PHARMACAL COMPANY
San Antonio, TX USA 78230 1355
Prenatal tablets manufactured by:
MISSION PHARMACAL COMPANY
San Antonio, TX USA 78230 1355
Copyright © 2020 Mission Pharmacal Company.
All rights reserved.
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CITRANATAL ASSURE
ascorbic acid, calcium citrate, iron, vitamin d, dl- alpha- tocopherol acetate, thiamine, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, iodine, zinc, copper, docusate sodium, doconexent and icosapent kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0178-0891 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0178-0891-30 30 in 1 CARTON 04/30/2014 1 1 in 1 BLISTER PACK; Type 1: Convenience Kit of Co-Package Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 Part 2 1 Part 1 of 2 PRENATAL VITAMIN
ascorbic acid, calcium citrate, iron, vitamin d, dl- alpha- tocopherol acetate, thiamine, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, iodine, zinc, copper and docusate sodium tablet, coatedProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 120 mg IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 35 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 400 [iU] ALPHA-TOCOPHEROL (UNII: H4N855PNZ1) (ALPHA-TOCOPHEROL - UNII:H4N855PNZ1) ALPHA-TOCOPHEROL 30 [iU] RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 3.4 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 20 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 25 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg IODINE (UNII: 9679TC07X4) (IODINE - UNII:9679TC07X4) IODINE 150 ug ZINC (UNII: J41CSQ7QDS) (ZINC - UNII:J41CSQ7QDS) ZINC 25 mg COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 2 mg DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg THIAMINE (UNII: X66NSO3N35) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 3 mg CALCIUM CITRATE (UNII: MLM29U2X85) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CITRATE ANHYDROUS 124 mg Inactive Ingredients Ingredient Name Strength POVIDONE K30 (UNII: U725QWY32X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MAGNESIUM SILICATE (UNII: 9B9691B2N9) MAGNESIUM STEARATE (UNII: 70097M6I30) SHELLAC (UNII: 46N107B71O) VANILLIN (UNII: CHI530446X) Product Characteristics Color white Score no score Shape OVAL Size 20mm Flavor Imprint Code 0891 Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/19/2008 Part 2 of 2 DHA
doconexent and icosapent capsuleProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCONEXENT (UNII: ZAD9OKH9JC) (DOCONEXENT - UNII:ZAD9OKH9JC) DOCONEXENT 300 mg ICOSAPENT (UNII: AAN7QOV9EA) (ICOSAPENT - UNII:AAN7QOV9EA) ICOSAPENT 0.75 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) SUNFLOWER OIL (UNII: 3W1JG795YI) GLYCERIN (UNII: PDC6A3C0OX) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) ROSEMARY (UNII: IJ67X351P9) TOCOPHEROL (UNII: R0ZB2556P8) ASCORBYL PALMITATE (UNII: QN83US2B0N) Product Characteristics Color orange (caramel color) Score no score Shape CAPSULE Size 23mm Flavor Imprint Code Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/19/2008 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/30/2014 Labeler - Mission Pharmacal Company (008117095) Registrant - Mission Pharmacal Company (927726893) Establishment Name Address ID/FEI Business Operations Mission Pharmacal Company 927726893 manufacture(0178-0891)


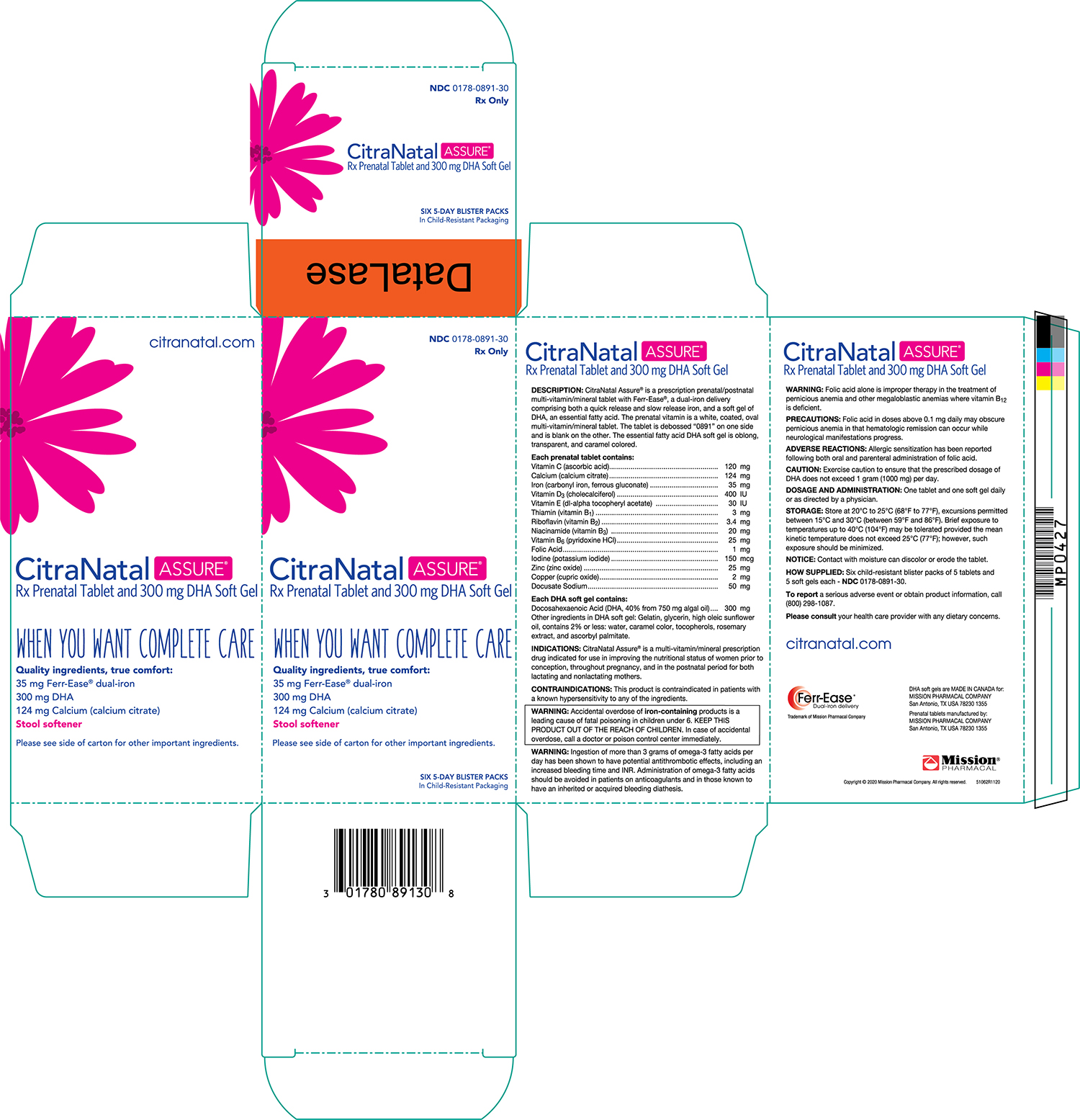

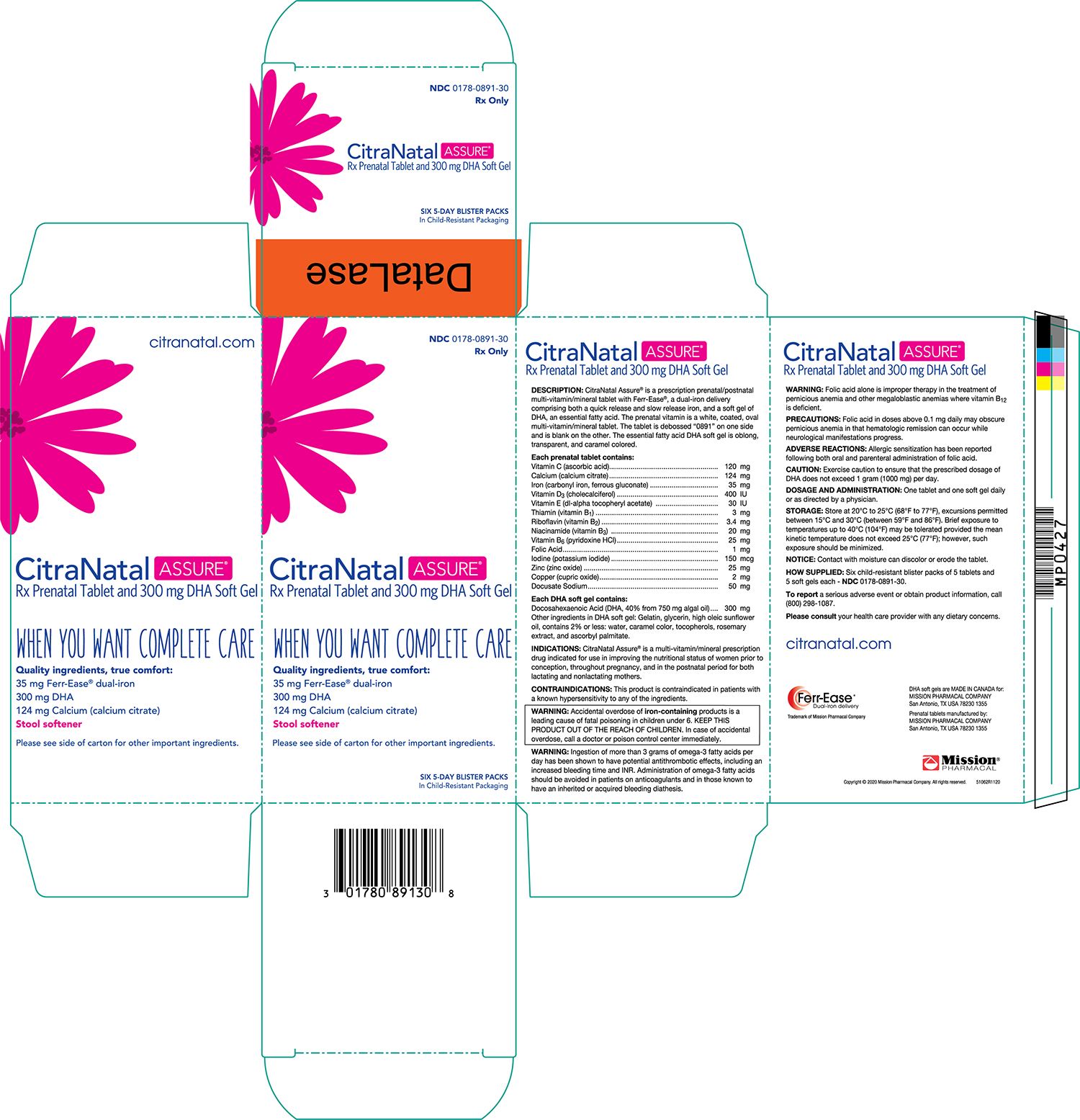 Retail Carton
Retail Carton