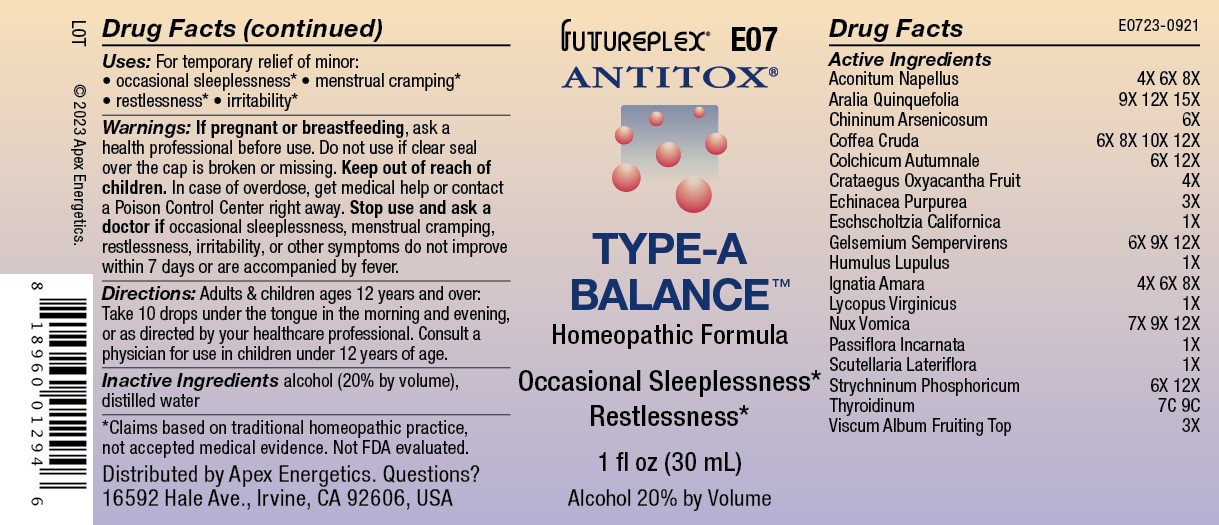
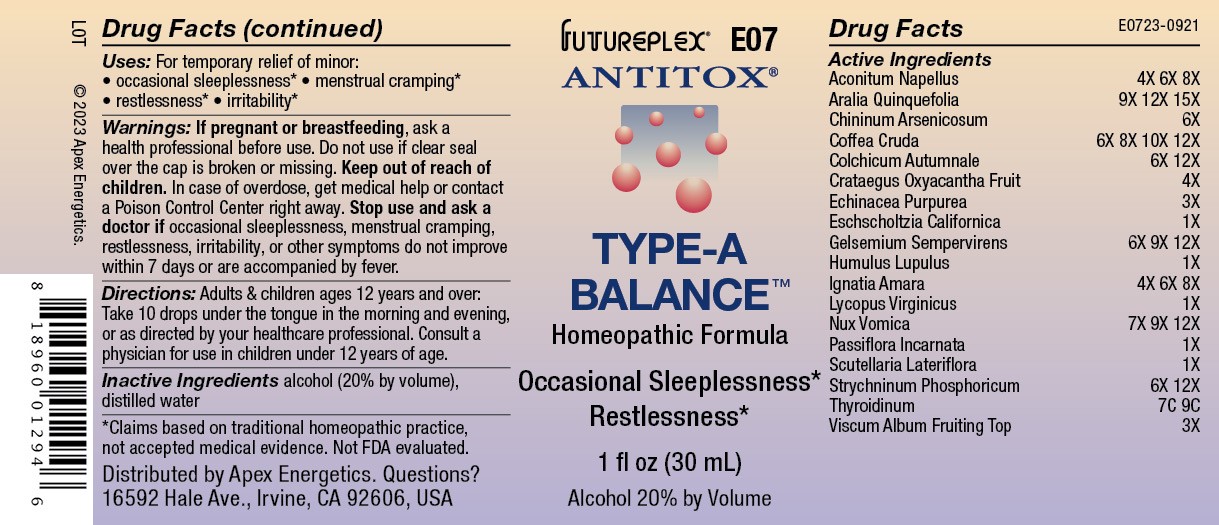
Label: E07 TYPE A BALANCE- aconitum napellus, aralia quinquefolia, chininum arsenicosum, coffea cruda, colchicum autumnale, crataegus oxyacantha fruit, echinacea purpurea, eschscholtzia californica, humulus lupulus, ignatia amara, lycopus virginicus, nux vomica, passiflora incarnata, scutellaria lateriflora, strychninum phosphoricum, thyroidinum, viscum album fruiting top, yellow jasmine root solution/ drops
- NDC Code(s): 63479-0507-1
- Packager: Apex Energetics Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients
Aconitum napellus
4X 6X 8X
Aralia quinquefolia
9X 12X 15X
Chininum arsenicosum
6X
Coffea cruda
6X 8X 10X 12X
Colchicum autumnale
6X 12X
Crataegus oxyacantha fruit
4X
Echinacea purpurea
3X
Eschscholtzia californica
1X
Gelsemium sempervirens
6X 9X 12X
Humulus lupulus
1X
Ignatia amara
4X 6X 8X
Lycopus virginicus
1X
Nux vomica
7X 9X 12X
Passiflora incarnata
1X
Scutellaria lateriflora
1X
Strychninum phosphoricum
6X 12X
Thyroidinum
7C 9C
Viscum album fruiting top
3X
- INDICATIONS & USAGE
- WARNINGS
- Directions:
- Inactive Ingredients
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
E07 TYPE A BALANCE
aconitum napellus, aralia quinquefolia, chininum arsenicosum, coffea cruda, colchicum autumnale, crataegus oxyacantha fruit, echinacea purpurea, eschscholtzia californica, humulus lupulus, ignatia amara, lycopus virginicus, nux vomica, passiflora incarnata, scutellaria lateriflora, strychninum phosphoricum, thyroidinum, viscum album fruiting top, yellow jasmine root solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63479-0507 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 8 [hp_X] in 1 mL LYCOPUS VIRGINICUS (UNII: TWH5125Q6F) (LYCOPUS VIRGINICUS - UNII:TWH5125Q6F) LYCOPUS VIRGINICUS 1 [hp_X] in 1 mL PASSIFLORA INCARNATA TOP (UNII: CLF5YFS11O) (PASSIFLORA INCARNATA TOP - UNII:CLF5YFS11O) PASSIFLORA INCARNATA TOP 1 [hp_X] in 1 mL SCUTELLARIA LATERIFLORA WHOLE (UNII: 7BP4DH5PDC) (SCUTELLARIA LATERIFLORA WHOLE - UNII:7BP4DH5PDC) SCUTELLARIA LATERIFLORA WHOLE 1 [hp_X] in 1 mL CRATAEGUS LAEVIGATA FRUIT (UNII: D5RZ7MF1YF) (CRATAEGUS LAEVIGATA FRUIT - UNII:D5RZ7MF1YF) CRATAEGUS LAEVIGATA FRUIT 4 [hp_X] in 1 mL COLCHICUM AUTUMNALE BULB (UNII: 993QHL78E6) (COLCHICUM AUTUMNALE BULB - UNII:993QHL78E6) COLCHICUM AUTUMNALE BULB 12 [hp_X] in 1 mL ARABICA COFFEE BEAN (UNII: 3SW678MX72) (ARABICA COFFEE BEAN - UNII:3SW678MX72) ARABICA COFFEE BEAN 12 [hp_X] in 1 mL QUININE ARSENITE (UNII: 42QO5P0NLM) (QUININE - UNII:A7V27PHC7A, ARSENITE ION - UNII:N5509X556J) QUININE ARSENITE 6 [hp_X] in 1 mL AMERICAN GINSENG (UNII: 8W75VCV53Q) (AMERICAN GINSENG - UNII:8W75VCV53Q) AMERICAN GINSENG 15 [hp_X] in 1 mL ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 8 [hp_X] in 1 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 12 [hp_X] in 1 mL THYROID, UNSPECIFIED (UNII: 0B4FDL9I6P) (THYROID, UNSPECIFIED - UNII:0B4FDL9I6P) THYROID, UNSPECIFIED 9 [hp_C] in 1 mL STRYCHNINE PHOSPHATE DIHYDRATE (UNII: TD67SYY51D) (STRYCHNINE - UNII:H9Y79VD43J) STRYCHNINE PHOSPHATE DIHYDRATE 12 [hp_X] in 1 mL VISCUM ALBUM FRUITING TOP (UNII: BK9092J5MP) (VISCUM ALBUM FRUITING TOP - UNII:BK9092J5MP) VISCUM ALBUM FRUITING TOP 3 [hp_X] in 1 mL GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 12 [hp_X] in 1 mL ECHINACEA PURPUREA (UNII: QI7G114Y98) (ECHINACEA PURPUREA - UNII:QI7G114Y98) ECHINACEA PURPUREA 3 [hp_X] in 1 mL ESCHSCHOLZIA CALIFORNICA (UNII: 9315HN272X) (ESCHSCHOLZIA CALIFORNICA - UNII:9315HN272X) ESCHSCHOLZIA CALIFORNICA 1 [hp_X] in 1 mL HOPS (UNII: 01G73H6H83) (HOPS - UNII:01G73H6H83) HOPS 1 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63479-0507-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 10/16/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/16/2023 Labeler - Apex Energetics Inc. (195816384)