Label: REVITADERM PSORIASIS- coal tar ointment
- NDC Code(s): 63347-112-01
- Packager: Blaine Labs Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 21, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients Section
- Purpose Section
- Keep Out of Reach Of Children Section
- Uses Section
- Warnings Section
- Directions Section
-
Inactive Ingredient Section
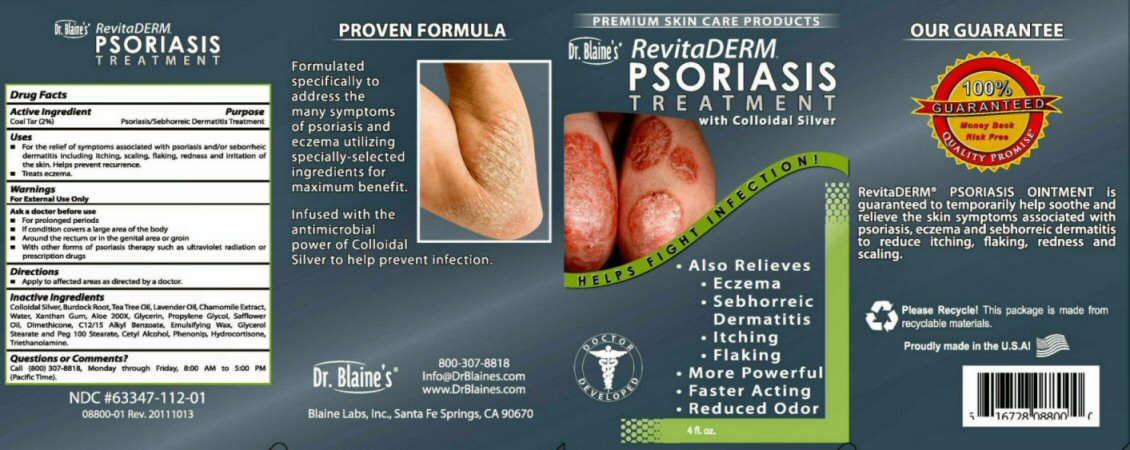
Inactive Ingredients Colloidal Silver, Burdock Root, Tea Tree Oil, Lavender Oil, Chamomile Extract, Water, Xanthan Gum, Aloe 200X, Glycerin, Propylene Glycol, Safflower Oil, Dimethicone, C12/15 Alky Benzoate, Emulsifying Wax, Glycerol Stearate and PEG 100 Stearate, Cetyl Alcohol, Phenonip (phenoxyethanol, methylparaben, butylparaben, ethylparaben, propylparaben), Hydrocortisone, Triethanolamine.
- Questions or Comments? Section
-
Package Label Section
Dr. Blaine's RevitaDERM PSORIASIS TREATMENT
NDC #63347-112-01 08800-01 Rev 20111013
PROVEN FORMULA
Formulated specifically to address the many symptoms of psoriasis and eczema utilizing specially selected ingredients for maximum benefit.
Infused with the antimicrobial power of Colloidal Silver to help prevent infection.
Dr. Blaine's 800 307-8818 info@DrBlaines.com www.DrBlaines.com Blaine Labs, Inc. Santa Fe Springs, CA 90670
PREMIUM SKIN CARE PRODUCTS HELPS FIGHT INFECTION
Also Relieves Eczema Sebhorreic Dermatitis Itching Flaking More Powerful Faster Acting Reduce Odor DOCTOR DEVELOPED
4 fl. oz
OUR GUARANTEE 100% GUARANTEED Money Back Rick Free QUALITY PROMISE
RevitaDerm PSORIIASIS OINTMENT is guaranteed to temporarily help soothe and relieve the kin symptoms associated with psoriasis, eczema, and sebhorreic dermatitis to reduce itching, flaking, redness, and scaling.
Please Recycle! This package is made from recyclable materails. Proudly made in the U.S.A.

res
-
INGREDIENTS AND APPEARANCE
REVITADERM PSORIASIS
coal tar ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63347-112 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COAL TAR (UNII: R533ESO2EC) (COAL TAR - UNII:R533ESO2EC) COAL TAR 2 mg in 100 mL Inactive Ingredients Ingredient Name Strength SILVER (UNII: 3M4G523W1G) ARCTIUM LAPPA ROOT (UNII: 597E9BI3Z3) TEA TREE OIL (UNII: VIF565UC2G) LAVENDER OIL (UNII: ZBP1YXW0H8) CHAMAEMELUM NOBILE FLOWER (UNII: O2T154T6OG) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) ALOE (UNII: V5VD430YW9) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SAFFLOWER OIL (UNII: 65UEH262IS) DIMETHICONE (UNII: 92RU3N3Y1O) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) CETYL ALCOHOL (UNII: 936JST6JCN) HYDROCORTISONE (UNII: WI4X0X7BPJ) TROLAMINE (UNII: 9O3K93S3TK) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) BUTYLPARABEN (UNII: 3QPI1U3FV8) ETHYLPARABEN (UNII: 14255EXE39) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63347-112-01 118 mL in 1 JAR; Type 0: Not a Combination Product 11/01/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 11/01/2011 Labeler - Blaine Labs Inc. (017314571) Registrant - Blaine Labs Inc. (017314571) Establishment Name Address ID/FEI Business Operations Blaine Labs Inc. 017314571 manufacture(63347-112)