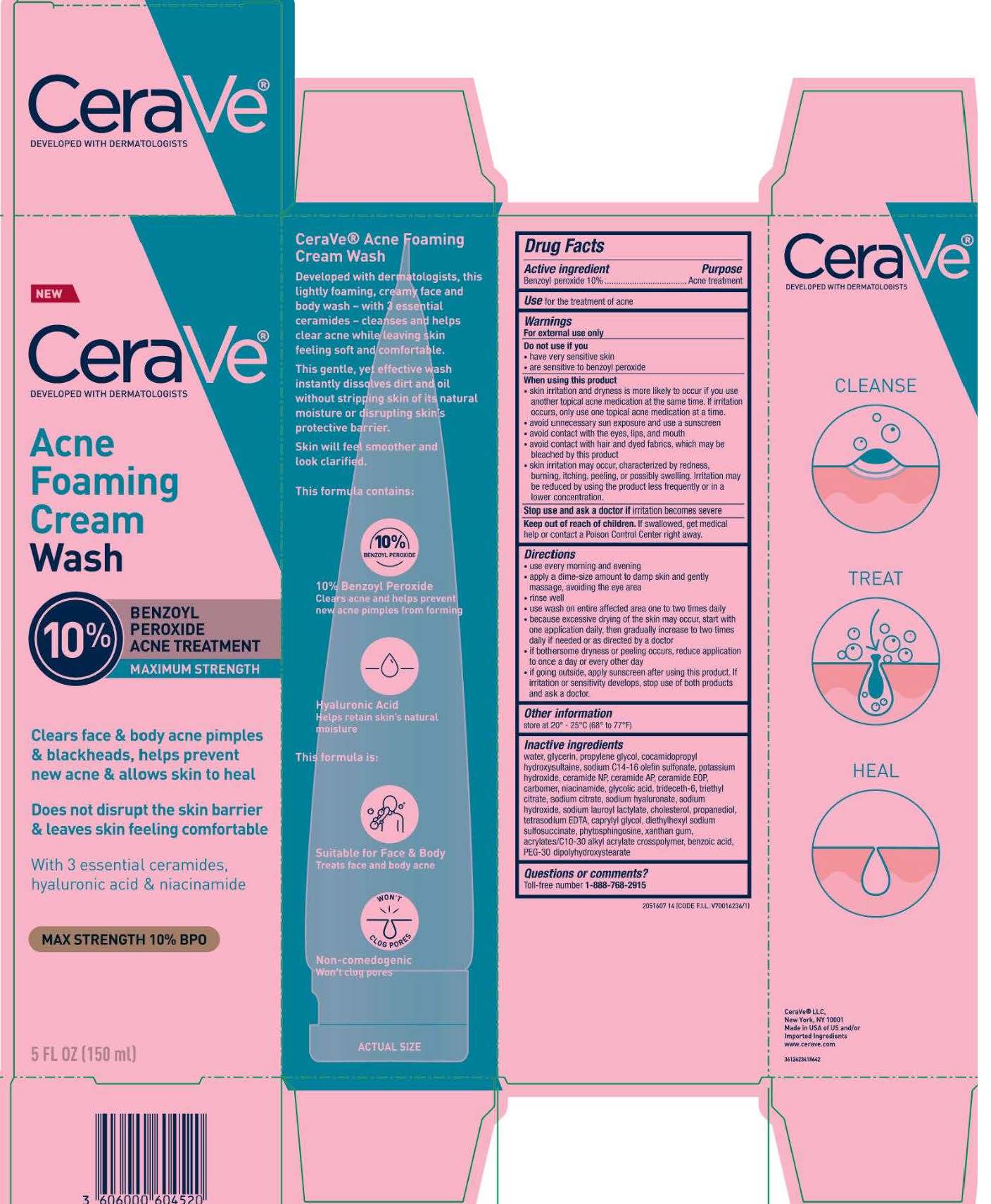
Label: CERAVE ACNE FOAMING CREAMWASH- benzoyl peroxide cream
- NDC Code(s): 49967-604-01, 49967-604-02
- Packager: L'Oreal USA Products Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Use
- Warnings
- Do not use if you
-
When using this product
• skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
• avoid unnecessary sun exposure and use a sunscreen
• avoid contact with the eyes, lips and mouth
• avoid contact with hair and dyed fabrics, which may be bleached by this product
• skin irritation may occur, characterized by redness, burning, itching, peeling or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration. - Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
• use every morning and evening
• apply a dime-size amount to damp skin and gently massage, avoiding the eye area
• rinse well
• use wash on entire affected area one to two times daily
• because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
• if bothersome dryness or peeling occurs, reduce application to once a day or every other day
• if going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor. -
Inactive ingredients
water, glycerin, propylene glycol, cocamidopropyl hydroxysultaine, sodium c14-16 olefin sulfonate, potassium hydroxide, ceramide NP, ceramide AP, ceramide EOP, carbomer, , niacinamide, glycolic acid, trideceth-6, triethyl citrate, sodium citrate, sodium hyaluronate, sodium hydroxide, sodium lauroyl lactylate, cholesterol, propanediol, tetrasodium EDTA, caprylyl glycol, diethylhexyl sodium sulfosuccinate, phytosphingosine, xanthan gum, acrylates/c10-30 alkyl acrylate crosspolymer, benzoic acid, PEG-30 dipolyhydroxystearate
- Other information
- Questions orcomments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CERAVE ACNE FOAMING CREAMWASH
benzoyl peroxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-604 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCAMIDOPROPYL HYDROXYSULTAINE (UNII: 62V75NI93W) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) CERAMIDE NP (UNII: 4370DF050B) CERAMIDE AP (UNII: F1X8L2B00J) CERAMIDE 1 (UNII: 5THT33P7X7) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) NIACINAMIDE (UNII: 25X51I8RD4) GLYCOLIC ACID (UNII: 0WT12SX38S) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRIDECETH-6 (UNII: 3T5PCR2H0C) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) SODIUM CITRATE (UNII: 1Q73Q2JULR) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) CHOLESTEROL (UNII: 97C5T2UQ7J) PROPANEDIOL (UNII: 5965N8W85T) EDETATE SODIUM (UNII: MP1J8420LU) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DOCUSATE SODIUM (UNII: F05Q2T2JA0) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) XANTHAN GUM (UNII: TTV12P4NEE) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) BENZOIC ACID (UNII: 8SKN0B0MIM) PEG-30 DIPOLYHYDROXYSTEARATE (UNII: 9713Q0S7FO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-604-01 1 in 1 CARTON 12/01/2023 1 150 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:49967-604-02 1 in 1 CARTON 12/01/2023 2 10 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/01/2023 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations Accupac, LLC 061595175 MANUFACTURE(49967-604)