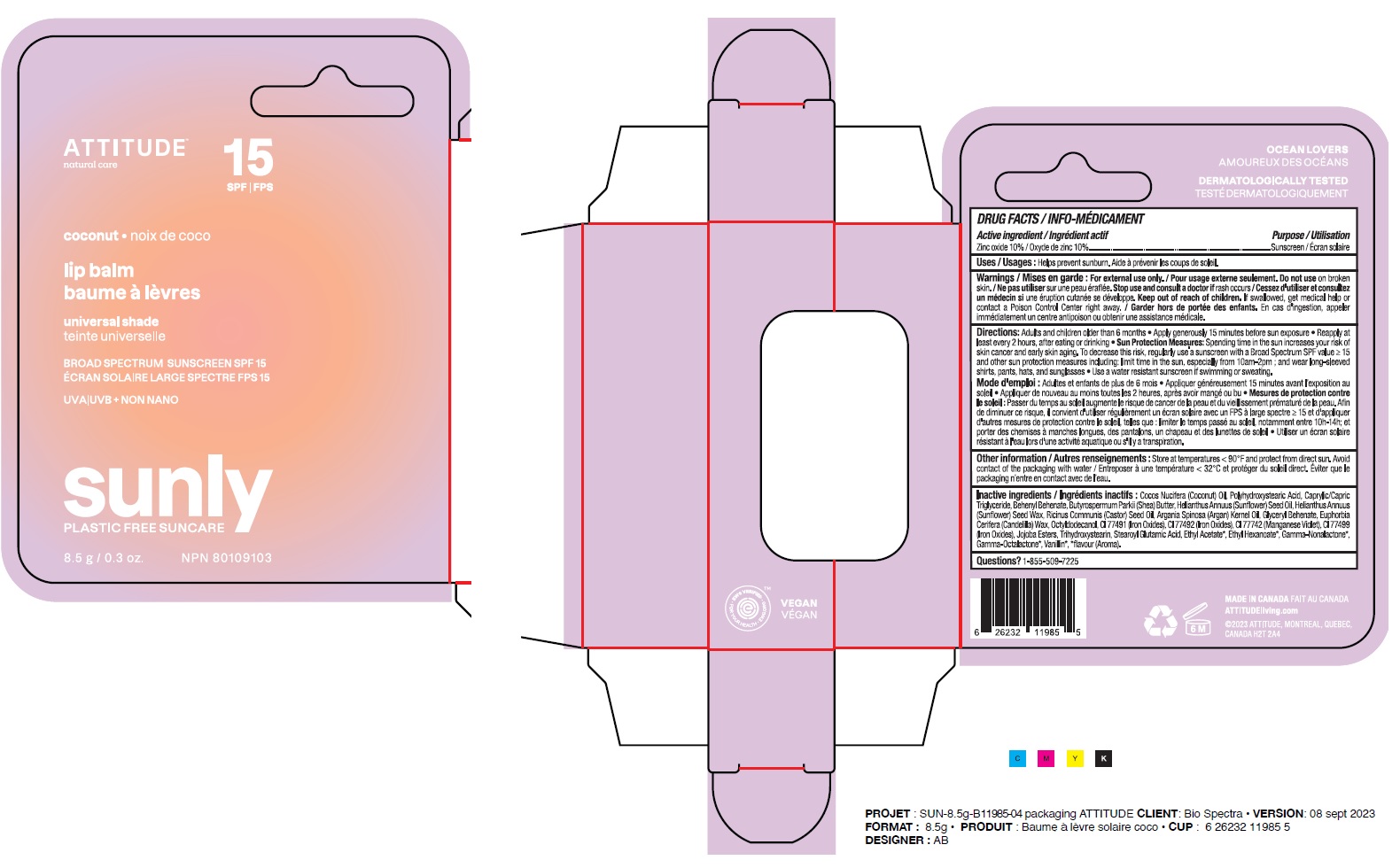
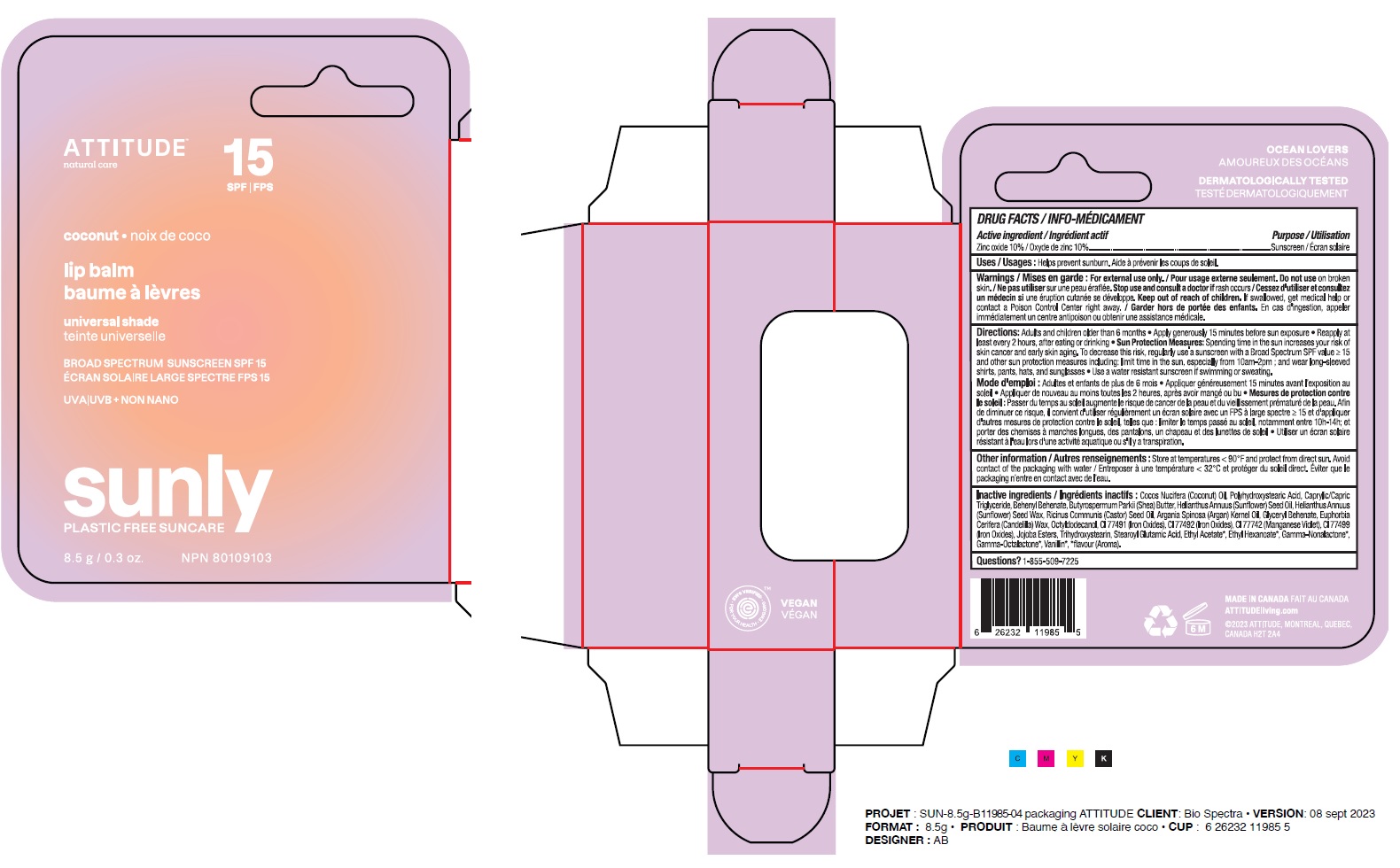
Label: ATTITUDE - LIP BALM - SPF 15 - COCONUT- zinc oxide stick
- NDC Code(s): 61649-985-01
- Packager: Attitude DBA 9055-7588 Québec Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Medicinal ingredient
- Purpose
- Uses
- Warnings
-
Directions
Adults and children older than 6 months.
- Apply generously 15 minutes before sun exposure.
- Reapply at least every 2 hours, after eating or drinking.
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPFvalue of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10am-2pm; and wear long-sleeved shirts, pants, hats and sunglasses.
- Use a water resistant sunscreen if swimming or sweating.
-
Inactive or non-medicinal ingredients
Cocos Nucifera (Coconut) Oil, Polyhydroxystearic Acid, Caprylic/Capric Triglyceride, Behenyl Behenate, Butyrospermum Parkii (Shea) Butter, Helianthus Annuus (Sunflower) Seed Oil, Helianthus Annuus (Sunflower) Seed Wax, Ricinus Communis (Castor) Seed Oil, Argania Spinosa (Argan) Kernel Oil, Glyceryl Behenate, Euphorbia Cerifera (Candelilla) Wax, Octyldodecanol, CI 77491 (Iron Oxides), CI 77492 (Iron Oxides), CI 77742 (Manganese Violet), CI 77499 (Iron Oxides), Jojoba Esters, Trihydroxystearin, Stearoyl Glutamic Acid, Ethyl Acetate*, Ethyl Hexanoate*, Gamma-Nonalactone*, Gamma-Octalactone*, Vanillin*, *flavour (Aroma).
- Other Information
- QUESTIONS
- PRINCIPAL DISPLAY PANEL - 8.5 g Tube Carton
-
INGREDIENTS AND APPEARANCE
ATTITUDE - LIP BALM - SPF 15 - COCONUT
zinc oxide stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61649-985 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 10 g in 100 g Inactive Ingredients Ingredient Name Strength FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) GLYCERYL MONOBEHENATE (UNII: A626UU0W2A) OCTYLDODECANOL (UNII: 461N1O614Y) COCONUT OIL (UNII: Q9L0O73W7L) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SHEA BUTTER (UNII: K49155WL9Y) SUNFLOWER OIL (UNII: 3W1JG795YI) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) BEHENYL BEHENATE (UNII: K8NU647RJ0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CASTOR OIL (UNII: D5340Y2I9G) ARGAN OIL (UNII: 4V59G5UW9X) CANDELILLA WAX (UNII: WL0328HX19) VANILLIN (UNII: CHI530446X) .GAMMA.-OCTALACTONE (UNII: UHD6M52X0K) .GAMMA.-NONALACTONE (UNII: I1XGH66S8P) ETHYL CAPROATE (UNII: FLO6YR1SHT) ETHYL ACETATE (UNII: 76845O8NMZ) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) STEAROYL GLUTAMIC ACID (UNII: 4R4O71786G) MANGANESE VIOLET (UNII: 72M48QQV8Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61649-985-01 8.5 g in 1 TUBE; Type 0: Not a Combination Product 02/15/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/15/2024 Labeler - Attitude DBA 9055-7588 Québec Inc. (201137051) Establishment Name Address ID/FEI Business Operations Attitude DBA 9055-7588 Québec Inc. 204307099 manufacture(61649-985) , label(61649-985) , pack(61649-985) , analysis(61649-985)