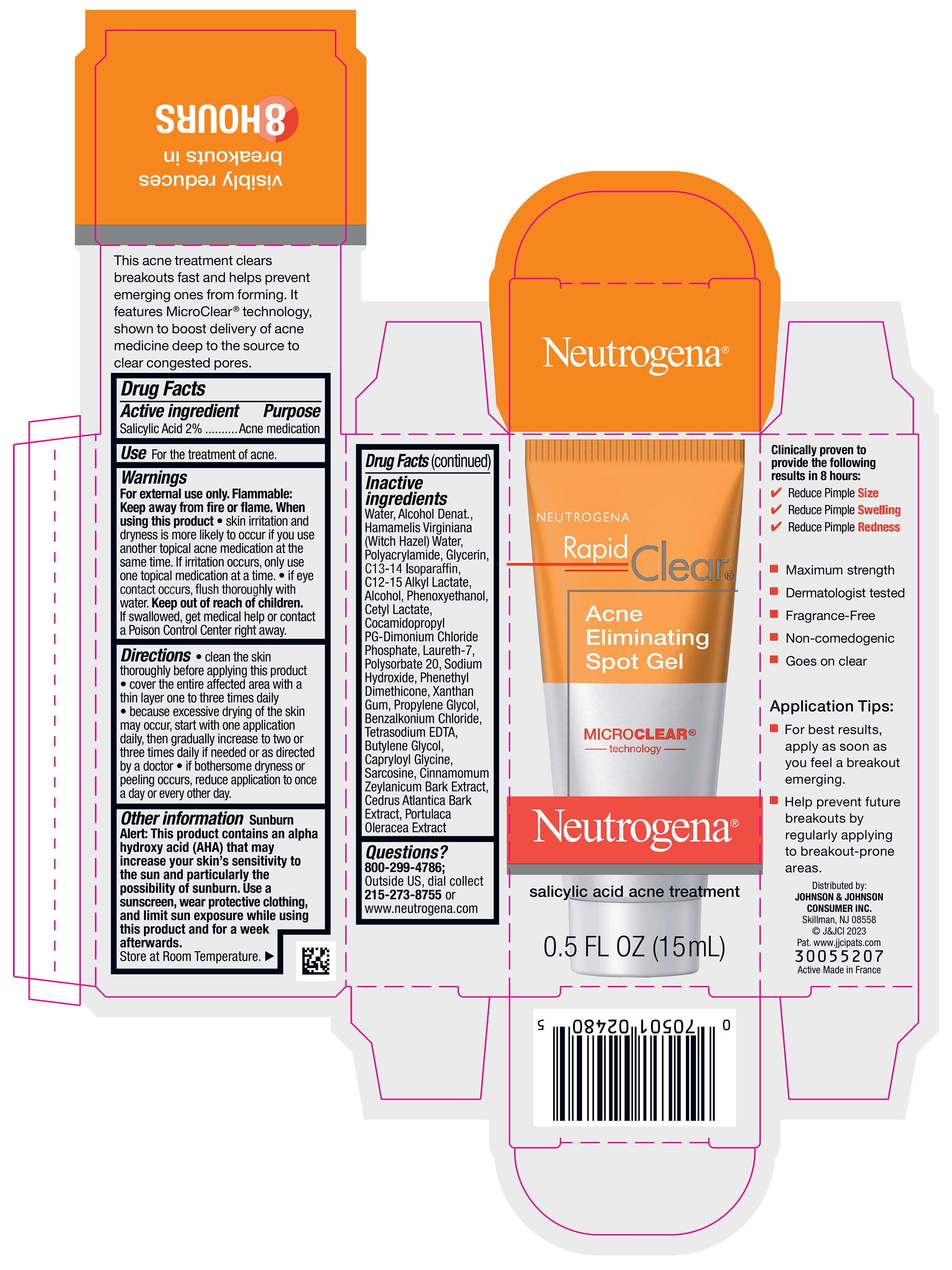
Label: NEUTROGENA RAPID CLEAR ACNE ELIMINATING SPOT- salicylic acid gel
- NDC Code(s): 69968-0825-1
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
-
Other information
Sunburn Alert
This product contains an alpha hydroxy acid (AHA) that may increase your skin’s sensitivity to the sun and particularly the possibility of sunburn. Use a sunscreen, wear protective clothing, and limit sun exposure while using this product and for a week afterwards.
Store at Room Temperature.
-
Inactive ingredients
Water, Alcohol Denat., Hamamelis Virginiana (Witch Hazel) Water, Polyacrylamide, Glycerin, C13-14 Isoparaffin, C12-15 Alkyl Lactate, Alcohol, Phenoxyethanol, Cetyl Lactate, Cocamidopropyl PG-Dimonium Chloride Phosphate, Laureth-7, Polysorbate 20, Sodium Hydroxide, Phenethyl Dimethicone, Xanthan Gum, Propylene Glycol, Benzalkonium Chloride, Tetrasodium EDTA, Butylene Glycol, Capryloyl Glycine, Sarcosine, Cinnamomum Zeylanicum Bark Extract, Cedrus Atlantica Bark Extract, Portulaca Oleracea Extract
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 15 mL Tube Carton
-
INGREDIENTS AND APPEARANCE
NEUTROGENA RAPID CLEAR ACNE ELIMINATING SPOT
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0825 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLOYL GLYCINE (UNII: 8TY5YO42NJ) SARCOSINE (UNII: Z711V88R5F) CINNAMON BARK OIL (UNII: XE54U569EC) CEDRUS ATLANTICA BARK (UNII: ITP1Q41UPF) PURSLANE (UNII: M6S840WXG5) WATER (UNII: 059QF0KO0R) HAMAMELIS VIRGINIANA TOP WATER (UNII: NT00Y05A2V) GLYCERIN (UNII: PDC6A3C0OX) SODIUM HYDROXIDE (UNII: 55X04QC32I) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) ALCOHOL (UNII: 3K9958V90M) PHENOXYETHANOL (UNII: HIE492ZZ3T) CETYL LACTATE (UNII: A7EVH2RK4O) COCAMIDOPROPYL PROPYLENE GLYCOL-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) LAURETH-7 (UNII: Z95S6G8201) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE SODIUM (UNII: MP1J8420LU) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0825-1 1 in 1 CARTON 08/30/2023 1 15 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 08/30/2023 Labeler - Johnson & Johnson Consumer Inc. (118772437)