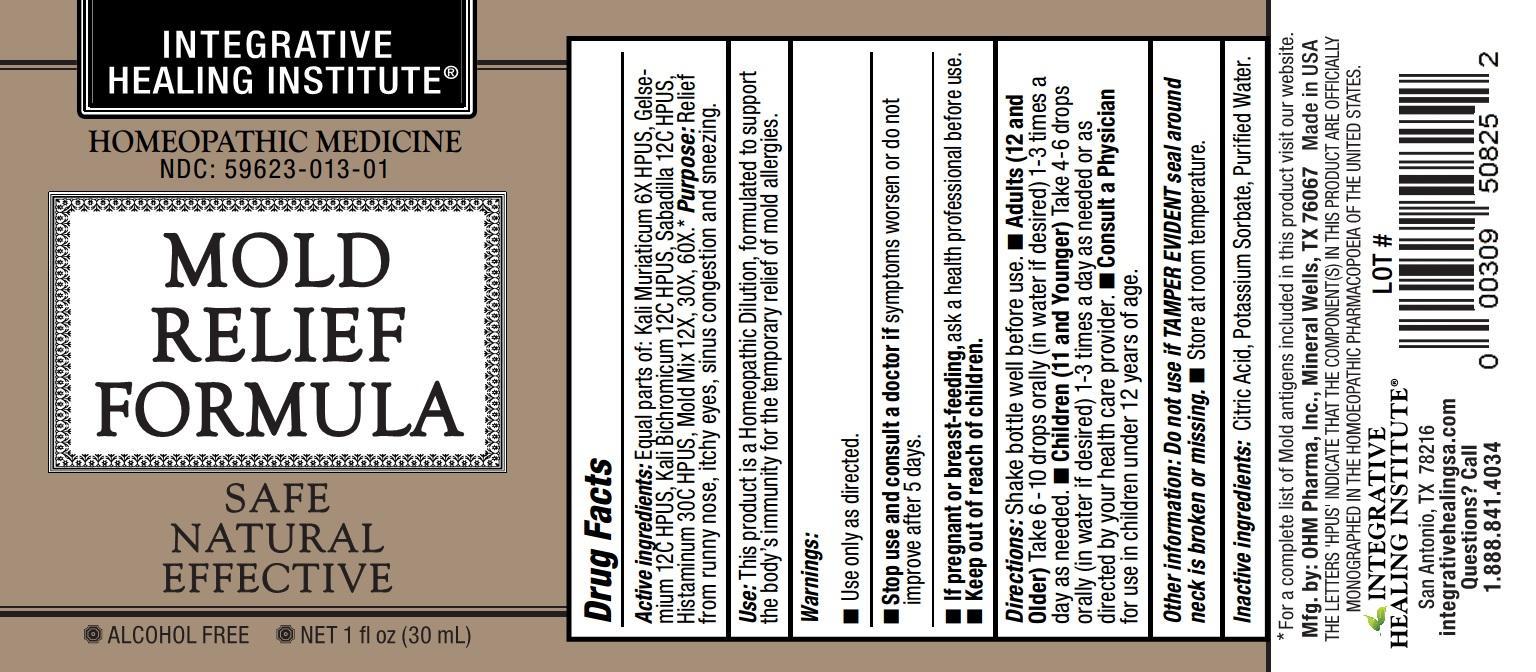
Label: MOLD RELIEF FORMULA- kali muriaticum, gelsemium, kali bichromicum, sabadilla, histaminum, mold mix. liquid
- NDC Code(s): 59623-013-01, 59623-013-02
- Packager: Integrative Healing Institute, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 29, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions:Shake bottle well before use.
- Adults (12 and Older) Take 6-10 drops orally (in water if desired) 1-3 times a day as needed.
- Children (11 and Younger) Take 4-6 drops orally (in water if desired) 1-3 times a day as needed or as directed by your health care provider.
- Consult a Physician for use in children under 12 years of age.
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MOLD RELIEF FORMULA
kali muriaticum, gelsemium, kali bichromicum, sabadilla, histaminum, mold mix. liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59623-013 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 6 [hp_X] in 1 mL GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 12 [hp_C] in 1 mL POTASSIUM DICHROMATE (UNII: T4423S18FM) (DICHROMATE ION - UNII:9LKY4BFN2V) POTASSIUM DICHROMATE 12 [hp_C] in 1 mL SCHOENOCAULON OFFICINALE SEED (UNII: 6NAF1689IO) (SCHOENOCAULON OFFICINALE SEED - UNII:6NAF1689IO) SCHOENOCAULON OFFICINALE SEED 12 [hp_C] in 1 mL HISTAMINE DIHYDROCHLORIDE (UNII: 3POA0Q644U) (HISTAMINE - UNII:820484N8I3) HISTAMINE DIHYDROCHLORIDE 30 [hp_C] in 1 mL ALTERNARIA ALTERNATA (UNII: 52B29REC7H) (ALTERNARIA ALTERNATA - UNII:52B29REC7H) ALTERNARIA ALTERNATA 12 [hp_X] in 1 mL ASPERGILLUS FUMIGATUS (UNII: X88DF51T48) (ASPERGILLUS FUMIGATUS - UNII:X88DF51T48) ASPERGILLUS FUMIGATUS 12 [hp_X] in 1 mL ASPERGILLUS NIGER VAR. NIGER (UNII: 9IOA40ANG6) (ASPERGILLUS NIGER VAR. NIGER - UNII:9IOA40ANG6) ASPERGILLUS NIGER VAR. NIGER 12 [hp_X] in 1 mL BOLETUS SATANAS FRUITING BODY (UNII: 68OG1407SM) (BOLETUS SATANAS FRUITING BODY - UNII:68OG1407SM) BOLETUS SATANAS FRUITING BODY 12 [hp_X] in 1 mL BOTRYTIS CINEREA (UNII: TBW53313S7) (BOTRYTIS CINEREA - UNII:TBW53313S7) BOTRYTIS CINEREA 12 [hp_X] in 1 mL CANDIDA ALBICANS (UNII: 4D7G21HDBC) (CANDIDA ALBICANS - UNII:4D7G21HDBC) CANDIDA ALBICANS 12 [hp_X] in 1 mL PASSALORA FULVA (UNII: HR6H5057CO) (PASSALORA FULVA - UNII:HR6H5057CO) PASSALORA FULVA 12 [hp_X] in 1 mL FUSARIUM OXYSPORUM (UNII: 5398RXP8KU) (FUSARIUM OXYSPORUM - UNII:5398RXP8KU) FUSARIUM OXYSPORUM 12 [hp_X] in 1 mL COCHLIOBOLUS SATIVUS (UNII: 3LN5B70U4W) (COCHLIOBOLUS SATIVUS - UNII:3LN5B70U4W) COCHLIOBOLUS SATIVUS 12 [hp_X] in 1 mL RHIZOPUS STOLONIFER (UNII: FEE198DK4Q) (RHIZOPUS STOLONIFER - UNII:FEE198DK4Q) RHIZOPUS STOLONIFER 12 [hp_X] in 1 mL AUREOBASIDIUM PULLULANS VAR. PULLUTANS (UNII: D1A2NG69CK) (AUREOBASIDIUM PULLULANS VAR. PULLUTANS - UNII:D1A2NG69CK) AUREOBASIDIUM PULLULANS VAR. PULLUTANS 12 [hp_X] in 1 mL USTILAGO MAYDIS (UNII: 4K7Z7K7SWG) (USTILAGO MAYDIS - UNII:4K7Z7K7SWG) USTILAGO MAYDIS 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59623-013-01 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 05/01/2016 2 NDC:59623-013-02 59 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 11/26/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/01/2016 Labeler - Integrative Healing Institute, LLC (938638595)