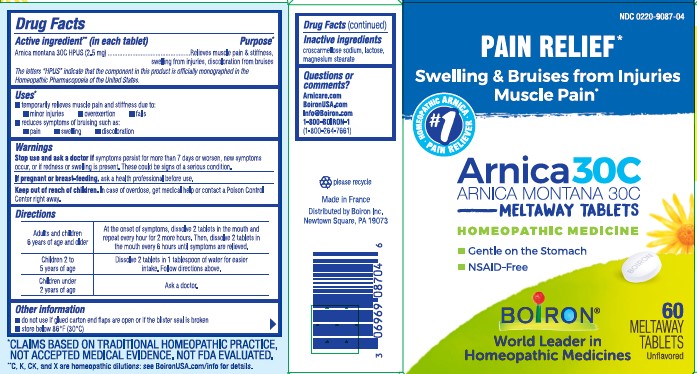
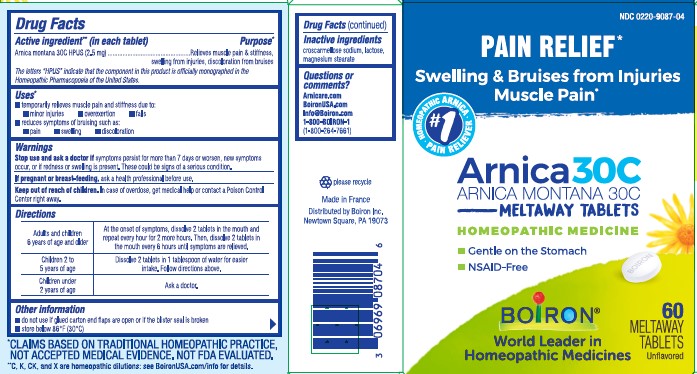
Label: ARNICA- arnica montana tablet
- NDC Code(s): 0220-9087-04
- Packager: Boiron
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated September 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Adults and children 6 years for age and older - At the onset of symptoms, dissolve 2 tablets in the mouth and repeat every hour for 2 more hours. Then, dissolve 2 tablets in the mouth every 6 hours until symptoms are relieved.
Children 2 to 5 years of age: Dissolve 2 tablets in 1 tablespoon of water for easier intake. Follow directions above.
Children under 2 years of age: Ask a doctor.
- INACTIVE INGREDIENT
- QUESTIONS
-
SPL UNCLASSIFIED SECTION
do not use if glued carton ends flaps are open or if the blister seal is broken
store below 86°F (30°C)
PAIN RELIEF*
Swelling & Bruises from Injuries Muscle Pain*
60 Meltaway Tablets, unflavored
Gentle on the stomach
NSAID-Free
No Known Drug Interactions
Non-Drowsy
Meltaway Tablets Require No Food or Water
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ARNICA
arnica montana tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0220-9087 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 30 [hp_C] Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) Product Characteristics Color white Score score with uneven pieces Shape ROUND (Boiron) Size 9mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0220-9087-04 60 in 1 BLISTER PACK; Type 0: Not a Combination Product 10/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/01/2023 Labeler - Boiron (282560473) Registrant - Boiron, Inc. (014892269) Establishment Name Address ID/FEI Business Operations Boiron 282560473 manufacture(0220-9087)