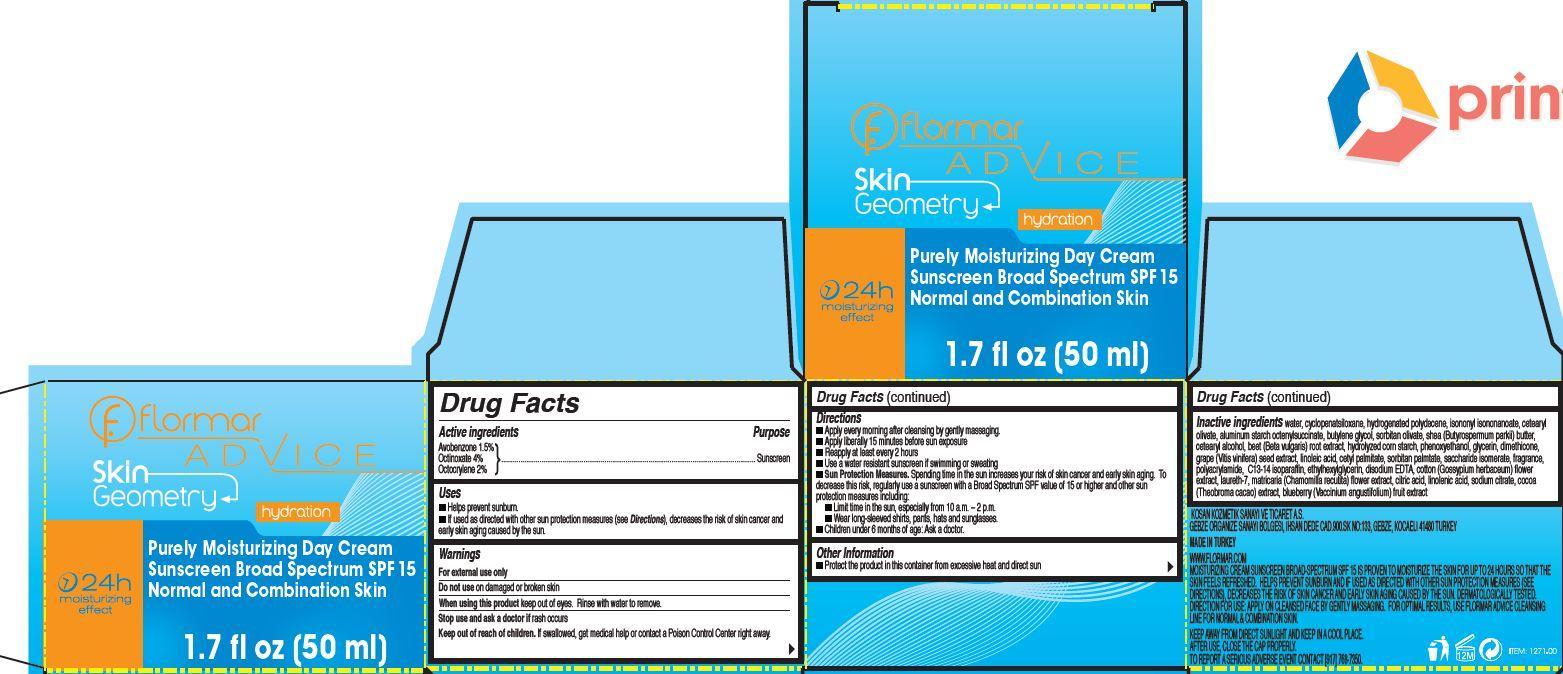
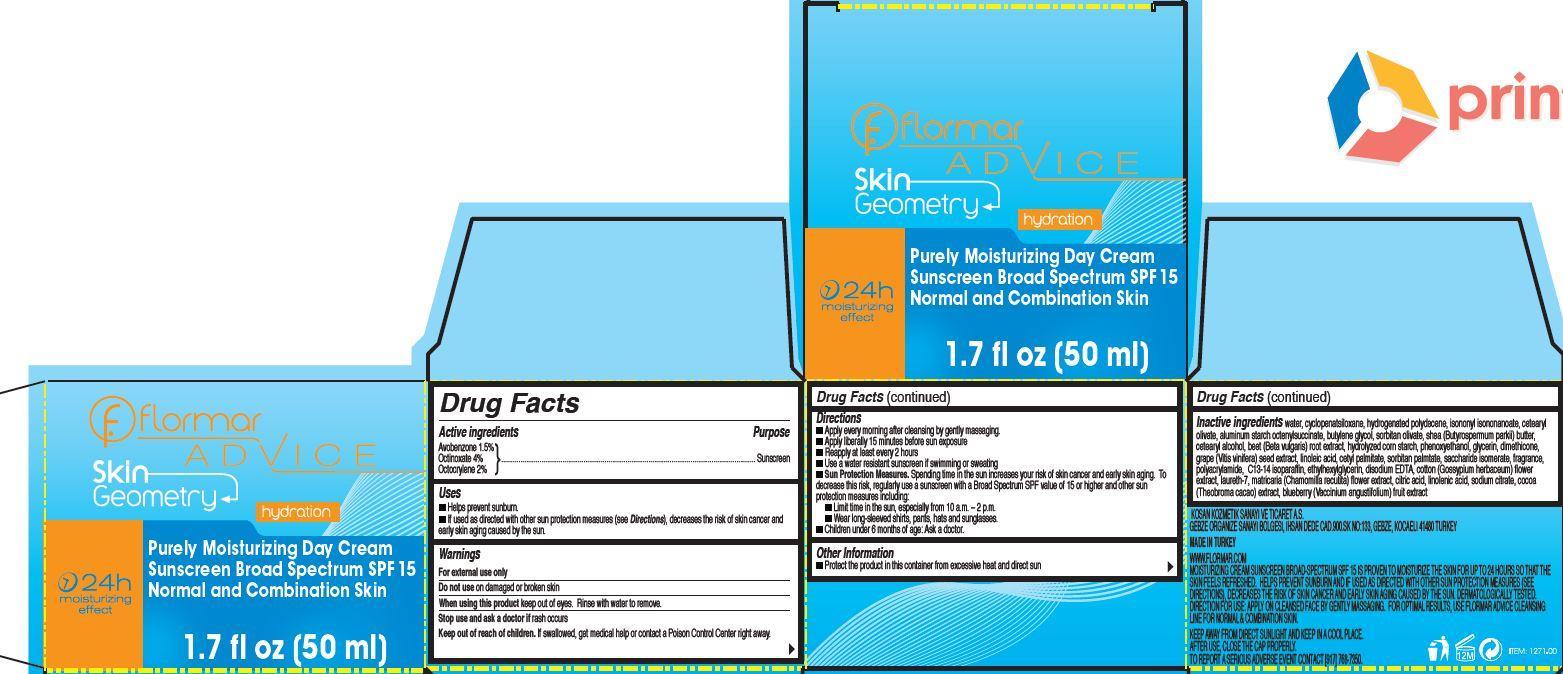
Label: FLORMAR ADVICE SKIN GEOMETRY PURELY MOISTURIZING DAY SUNSCREEN BROAD SPECTRUM SPF 15 NORMAL AND COMBINATION SKIN- avobenzone, octinoxate, octocrylene cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 61722-022-00 - Packager: Kosan Kozmetik Sanayi ve Ticaret A.S.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 18, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- flormar ADVICE Skin Geometry Purely Moisturizing Day Cream Sunscreen Broad Spectrum SPF 15 Normal and Combination Skin
- Active ingredients
- Uses
- Warnings
-
Directions
- Apply on cleansed face, neck and neckline by gently massaging every morning.
- Apply liberally 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. – 2 p.m.
- Wear long-sleeved shirts, pants, hats and sunglasses.
- Children under 6 months of age: Ask a doctor.
- Other Information
-
Inactive ingredients
water, cyclopenatsiloxane, hydrogenated polydecene, isononyl isononanoate, cetearyl olivate, aluminum starch octenylsuccinate, butylene glycol, sorbitan olivate, shea (Butyrospermum parkii) butter, cetearyl alcohol, beet (Beta vulgaris) root extract, hydrolyzed corn starch, phenoxyethanol, glycerin, dimethicone, grape (Vitis vinifera) seed extract, linoleic acid, cetyl palmitate, sorbitan palmtate, saccharide isomerate, fragrance, polyacrylamide, C13-14 isoparaffin, ethylhexylglycerin, disodium EDTA, cotton (Gossypium herbaceum) flower extract, laureth-7, matricaria (Chamomilla recutita) flower extract, citric acid, linolenic acid, sodium citrate, cocoa (Theobroma cacao) extract, blueberry (Vaccinium angustifolium) fruit extract
- flormar ADVICE Skin Geometry Purely Moisturizing Day Cream Sunscreen Broad Spectrum SPF 15 Normal and Combination Skin 50ml (61722-022-00)
-
INGREDIENTS AND APPEARANCE
FLORMAR ADVICE SKIN GEOMETRY PURELY MOISTURIZING DAY SUNSCREEN BROAD SPECTRUM SPF 15 NORMAL AND COMBINATION SKIN
avobenzone, octinoxate, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61722-022 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 15 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 40 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CETEARYL OLIVATE (UNII: 58B69Q84JO) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SORBITAN OLIVATE (UNII: MDL271E3GR) SHEA BUTTER (UNII: K49155WL9Y) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BEET (UNII: N487KM8COK) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) VITIS VINIFERA SEED (UNII: C34U15ICXA) LINOLEIC ACID (UNII: 9KJL21T0QJ) CETYL PALMITATE (UNII: 5ZA2S6B08X) SORBITAN MONOPALMITATE (UNII: 77K6Z421KU) SACCHARIDE ISOMERATE (UNII: W8K377W98I) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) EDETATE DISODIUM (UNII: 7FLD91C86K) LEVANT COTTON SEED (UNII: 550E4N439V) LAURETH-7 (UNII: Z95S6G8201) CHAMOMILE (UNII: FGL3685T2X) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) LINOLENIC ACID (UNII: 0RBV727H71) SODIUM CITRATE (UNII: 1Q73Q2JULR) COCOA (UNII: D9108TZ9KG) VACCINIUM ANGUSTIFOLIUM WHOLE (UNII: R3538BZ1BW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61722-022-00 1 in 1 BOX 06/16/2014 1 50 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/16/2014 Labeler - Kosan Kozmetik Sanayi ve Ticaret A.S. (644090409)