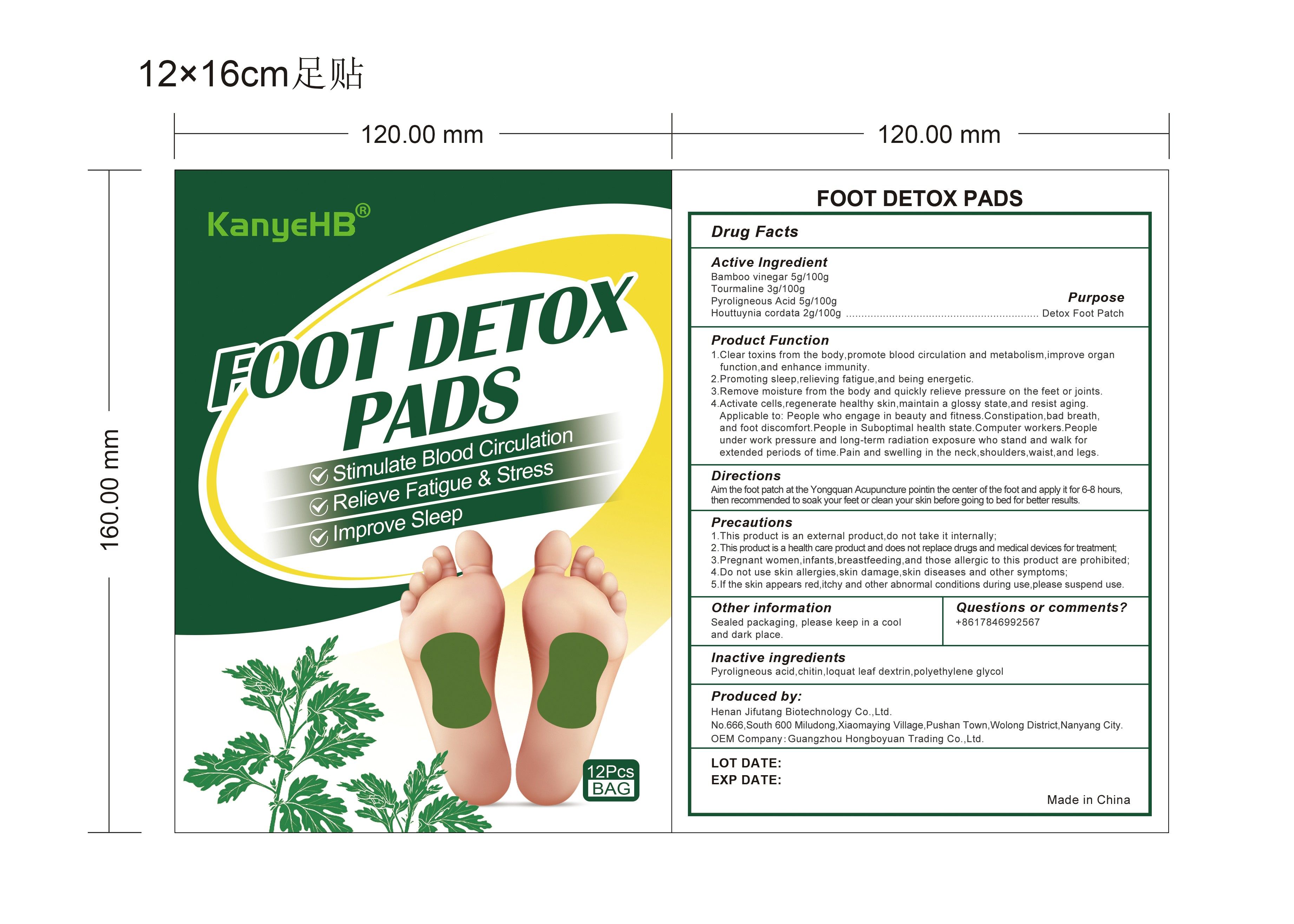
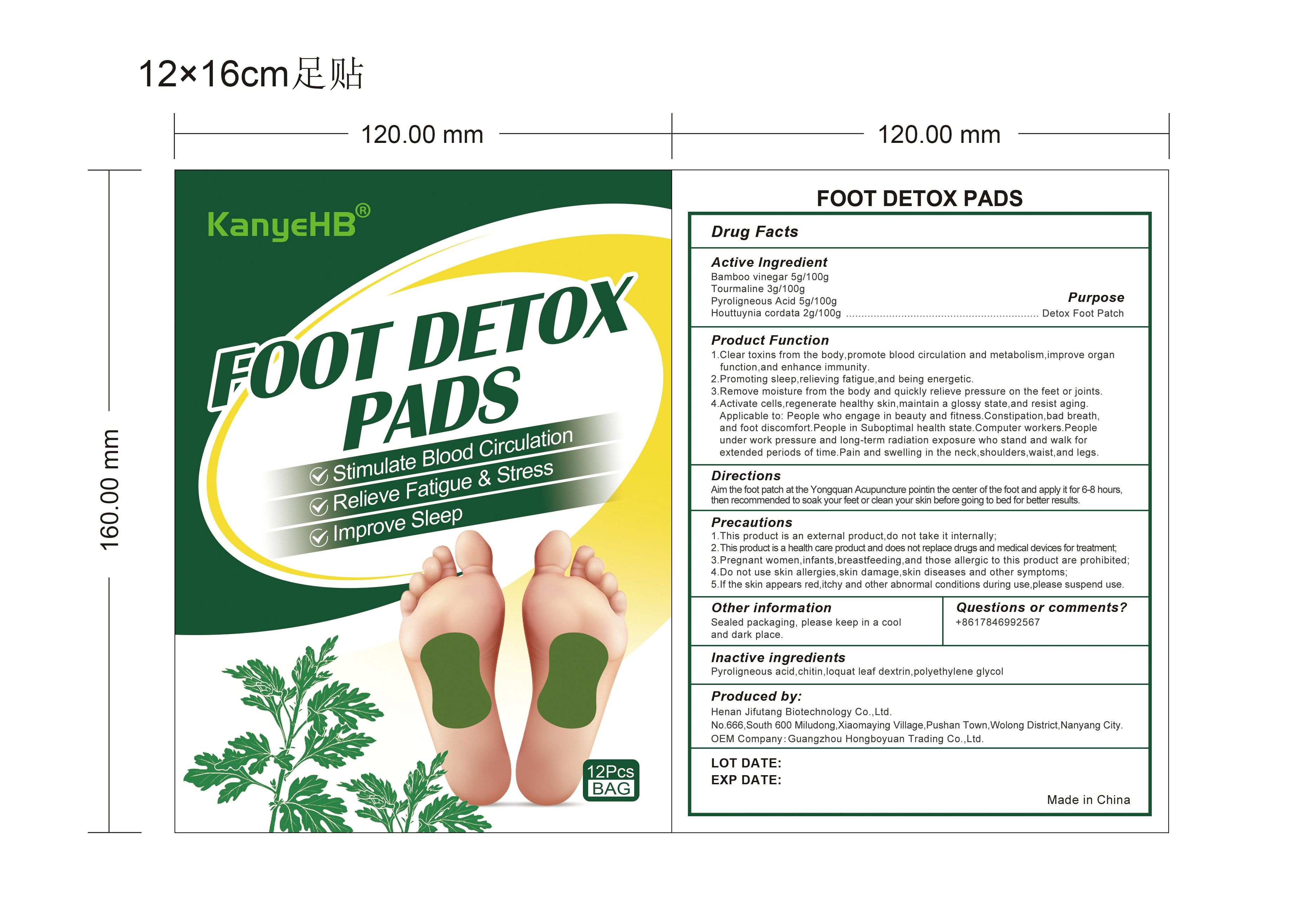
Label: FOOT DETOX PADS- bamboo vinegar, tourmaline, pyroligneous acid, houttuynia cordata patch
- NDC Code(s): 83713-004-11, 83713-004-12
- Packager: Guangzhou Hongboyuan Trading Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
-
INDICATIONS & USAGE
Product Function
1. Clear toxins from the body,promote blood circulation and metabolism, improve organ function, and enhance immunity.
2. Promoting sleep, relieving fatigue, and being energetic.
3. Remove moisture from the body and quickly relieve pressure on the feet or joints.
4. Activate cells, regenerate healthy skin, maintain a glossy state, and resist aging. Applicable to:People who engage in beauty and fitness. Constipation, bad breath. and foot discomfortPeople in Suboptimal health state. Computer workers. People under work pressure and long-term radiation exposure who stand and walk for extended periods of time. Pain and swelling in the neck, shoulders, waist, and legs.
- DOSAGE & ADMINISTRATION
-
WARNINGS
Precautions:
1. This product is an external product, do not take it internally;
2. This product is a health care product and does notreplace drugs and medical devices for treatment;
3. Pregnant women, infants, breastfeeding, and those allergic to this product are prohibited;
4. Do not use skin allergies, skin damage, skin diseases and other symptoms;
5. If the skin appears red, itchy and other abnormal conditions during use, please suspend use.
- KEEP OUT OF REACH OF CHILDREN
- QUESTIONS
- INACTIVE INGREDIENT
- STATEMENT OF IDENTITY
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FOOT DETOX PADS
bamboo vinegar, tourmaline, pyroligneous acid, houttuynia cordata patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83713-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BAMBUSA BAMBOS SAP (UNII: DPQ0BJD6MR) (BAMBUSA BAMBOS SAP - UNII:DPQ0BJD6MR) BAMBUSA BAMBOS SAP 5 g in 100 g SCHORL TOURMALINE (UNII: 173O8XLY6T) (SCHORL TOURMALINE - UNII:173O8XLY6T) SCHORL TOURMALINE 3 g in 100 g HOUTTUYNIA CORDATA WHOLE (UNII: O3E12ZLW5T) (HOUTTUYNIA CORDATA WHOLE - UNII:O3E12ZLW5T) HOUTTUYNIA CORDATA WHOLE 2 g in 100 g PYROLIGNEOUS ACID (UNII: N4G9GAT76C) (PYROLIGNEOUS ACID - UNII:N4G9GAT76C) PYROLIGNEOUS ACID 5 g in 100 g Inactive Ingredients Ingredient Name Strength CHITIN (UNII: 8SH93A7QWW) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83713-004-12 12 in 1 BAG 06/01/2023 1 NDC:83713-004-11 2.1 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/01/2023 Labeler - Guangzhou Hongboyuan Trading Co., Ltd. (723797279) Registrant - Henan Jifutang Biotechnology Co., Ltd. (848209767) Establishment Name Address ID/FEI Business Operations Henan Jifutang Biotechnology Co., Ltd. 848209767 label(83713-004) , manufacture(83713-004)