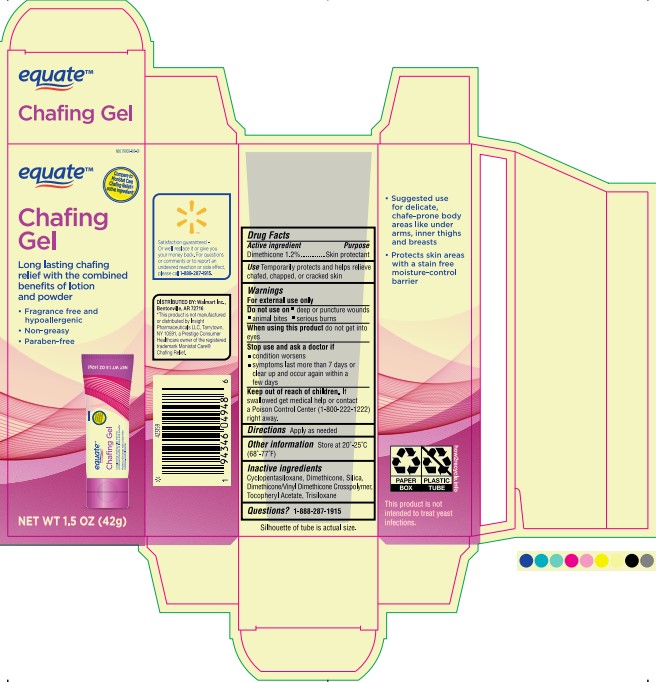
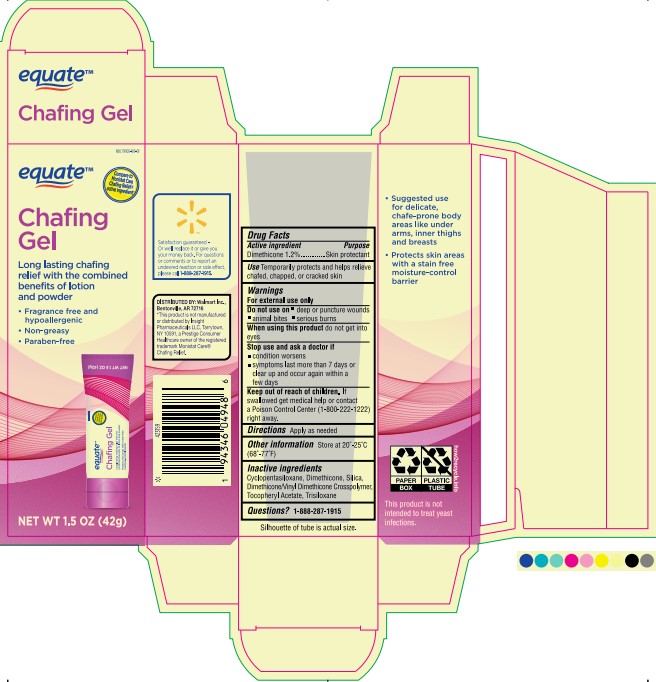
Label: EQUATE CHAFING GEL- dimethicone gel
- NDC Code(s): 79903-169-01
- Packager: Wal-Mart Stores Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- WARNINGS
- ASK DOCTOR
- DO NOT USE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- QUESTIONS
- STOP USE
- WHEN USING
- STORAGE AND HANDLING
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EQUATE CHAFING GEL
dimethicone gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-169 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE 200 (UNII: RGS4T2AS00) (DIMETHICONE 200 - UNII:RGS4T2AS00) DIMETHICONE 200 0.504 g in 42 g Inactive Ingredients Ingredient Name Strength DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) DIMETHICONE (UNII: 92RU3N3Y1O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 0.000042 g in 42 g TRISILOXANE (UNII: 9G1ZW13R0G) 0.000042 g in 42 g DIMETHICONE 500 (UNII: 5L1VVC3K8O) 0.126 g in 42 g CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) 1.26 g in 42 g Product Characteristics Color gray (Pale Gray, translucent) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-169-01 1 in 1 CARTON 06/28/2023 1 42 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 06/28/2023 Labeler - Wal-Mart Stores Inc (051957769)