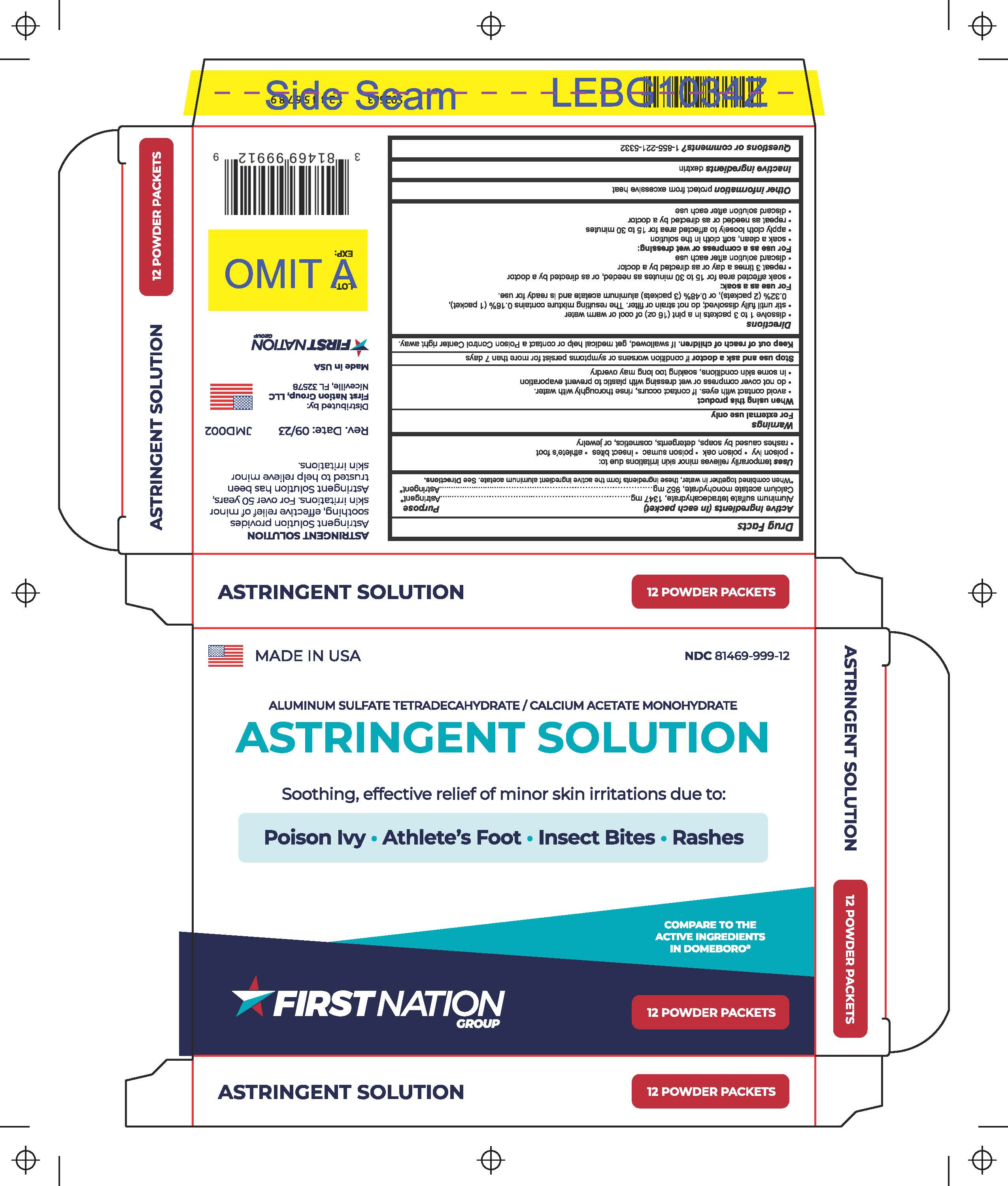
Label: ASTRINGENT SOLUTION- aluminum sulfate tetradecahydrate, calcium acetate monohydrate powder, for solution
- NDC Code(s): 81469-999-01, 81469-999-12
- Packager: First Nation Group
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- PURPOSE
- WARNINGS
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- Dissolve 1 to 3 packets in a pint (16 oz) of cool or warm water.
- Stir until fully dissolved; do not strain or filter. The resulting mixture contains 0.16% (1 packet), 0.32% (2 packets), or 0.48% (3packets) aluminum acetate and is ready for use.
For use as a soak:
- Soak affected area for 15 to 30 minutes as needed, or as directed by a doctor.
- Repeat 3 times a day or as directed by a doctor.
- Discard solution after each use.
For use as a compress or wet dressing:
- Soak a clean, soft cloth in the solution.
- Apply cloth loosely to affected area for 15 to 30 minutes.
- Repeat as needed or as directed by a doctor.
- Discard solution after each use.
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ASTRINGENT SOLUTION
aluminum sulfate tetradecahydrate, calcium acetate monohydrate powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81469-999 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM ACETATE MONOHYDRATE (UNII: 7ZA48GIM5H) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 952 mg in 2299 mg ALUMINUM SULFATE TETRADECAHYDRATE (UNII: E3UT66504P) (ALUMINUM CATION - UNII:3XHB1D032B) ALUMINUM CATION 1347 mg in 2299 mg Inactive Ingredients Ingredient Name Strength ICODEXTRIN (UNII: 2NX48Z0A9G) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81469-999-12 12 in 1 BOX 09/27/2023 1 NDC:81469-999-01 2299 mg in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part347 09/27/2023 Labeler - First Nation Group (078875731)