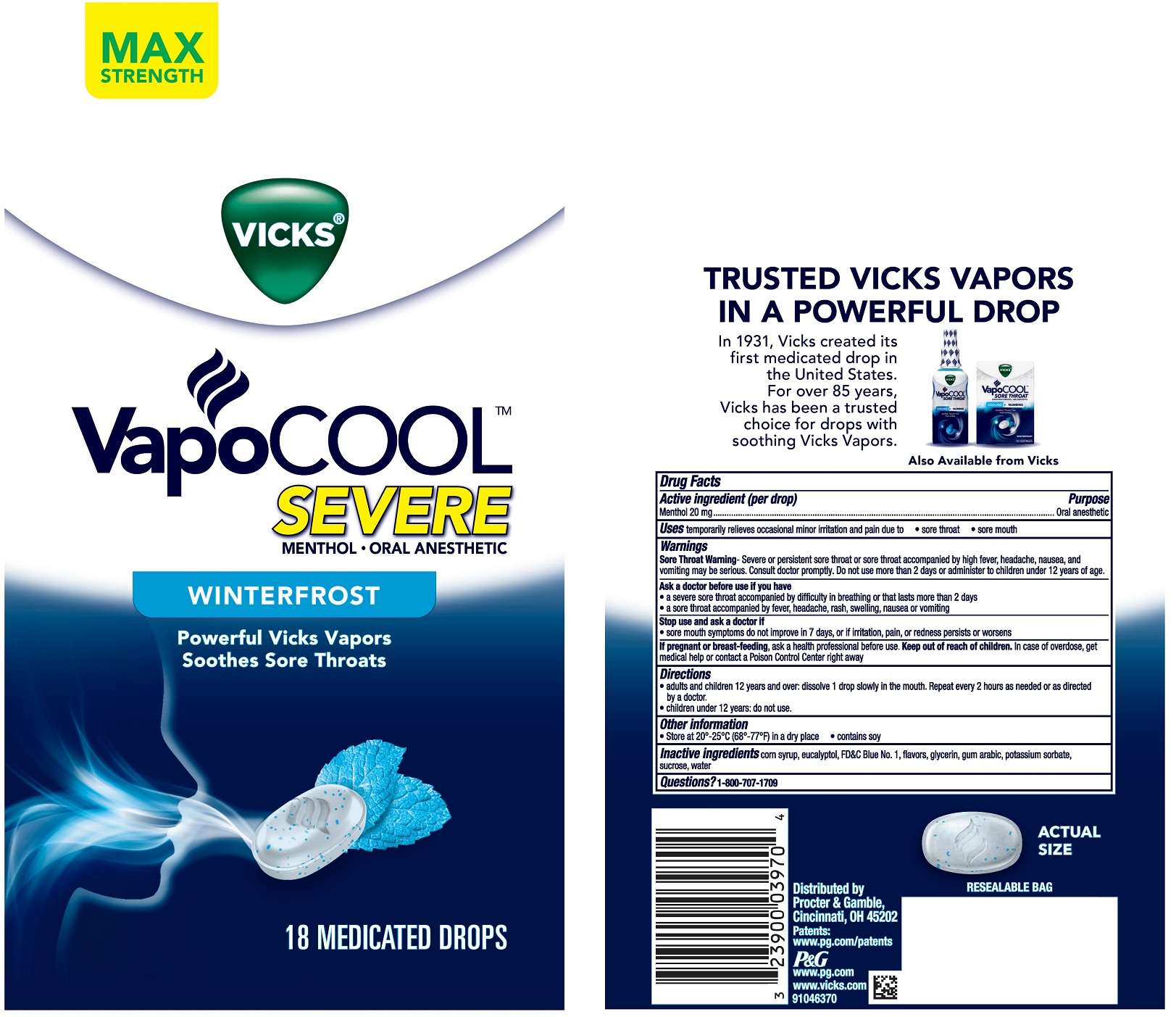
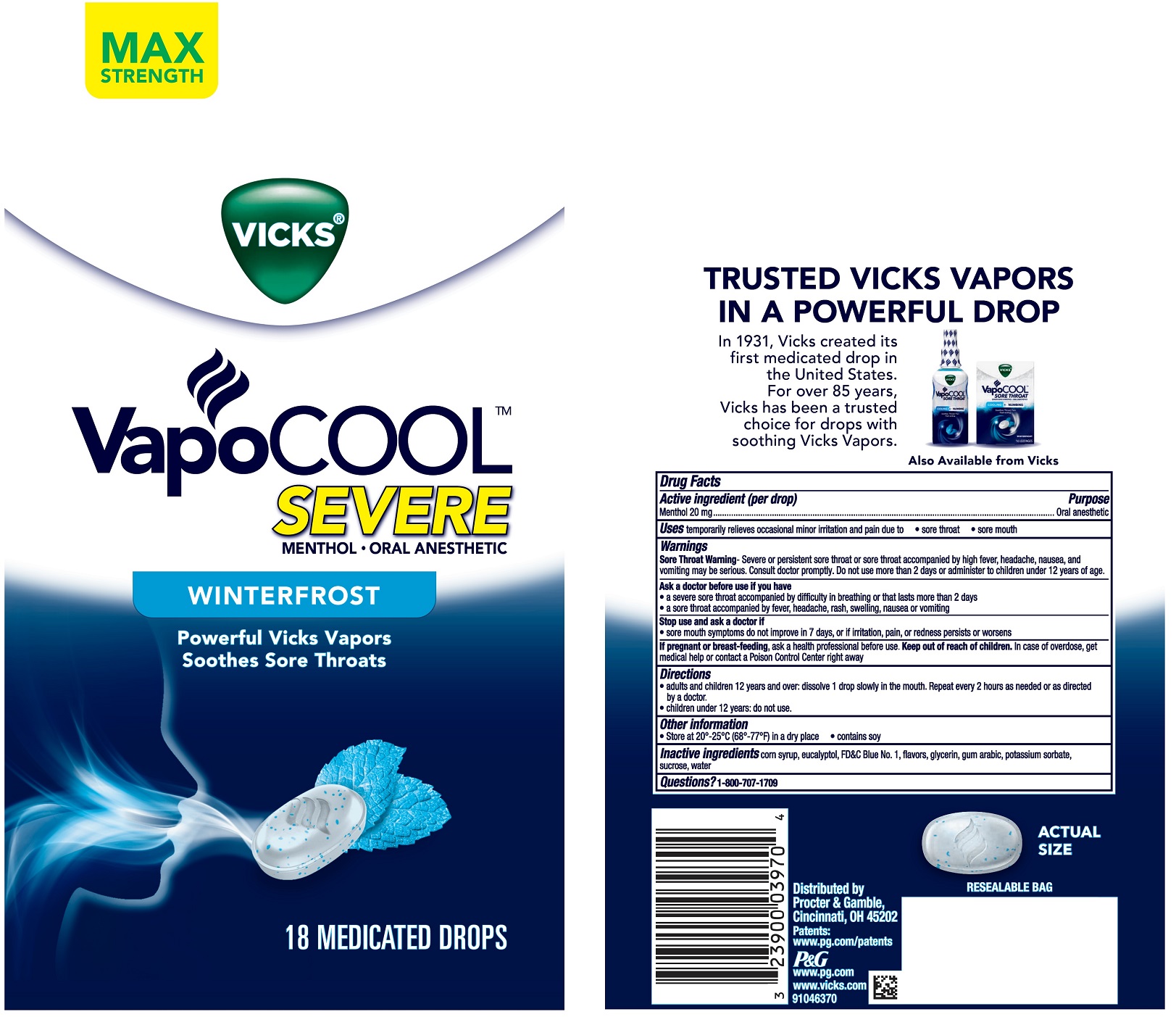
Label: VICKS VAPOCOOL SEVERE WINTERFROST- menthol lozenge
- NDC Code(s): 37000-597-18, 37000-597-20, 37000-597-45
- Packager: The Procter & Gamble Manufacturing Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (per drop)
- Purpose
- Uses
- Warnings
- Ask a doctor before use if you have
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VICKS VAPOCOOL SEVERE WINTERFROST
menthol lozengeProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37000-597 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 20 mg Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) ACACIA (UNII: 5C5403N26O) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) GLYCERIN (UNII: PDC6A3C0OX) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) CORN SYRUP (UNII: 9G5L16BK6N) EUCALYPTOL (UNII: RV6J6604TK) WATER (UNII: 059QF0KO0R) Product Characteristics Color white (with blue specks) Score no score Shape OVAL Size 23mm Flavor MENTHOL Imprint Code vapor Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37000-597-18 18 in 1 BAG 12/29/2017 1 1 in 1 POUCH; Type 0: Not a Combination Product 2 NDC:37000-597-45 45 in 1 BAG 12/29/2017 2 1 in 1 POUCH; Type 0: Not a Combination Product 3 NDC:37000-597-20 200 in 1 BAG 07/09/2019 3 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 12/29/2017 Labeler - The Procter & Gamble Manufacturing Company (004238200)