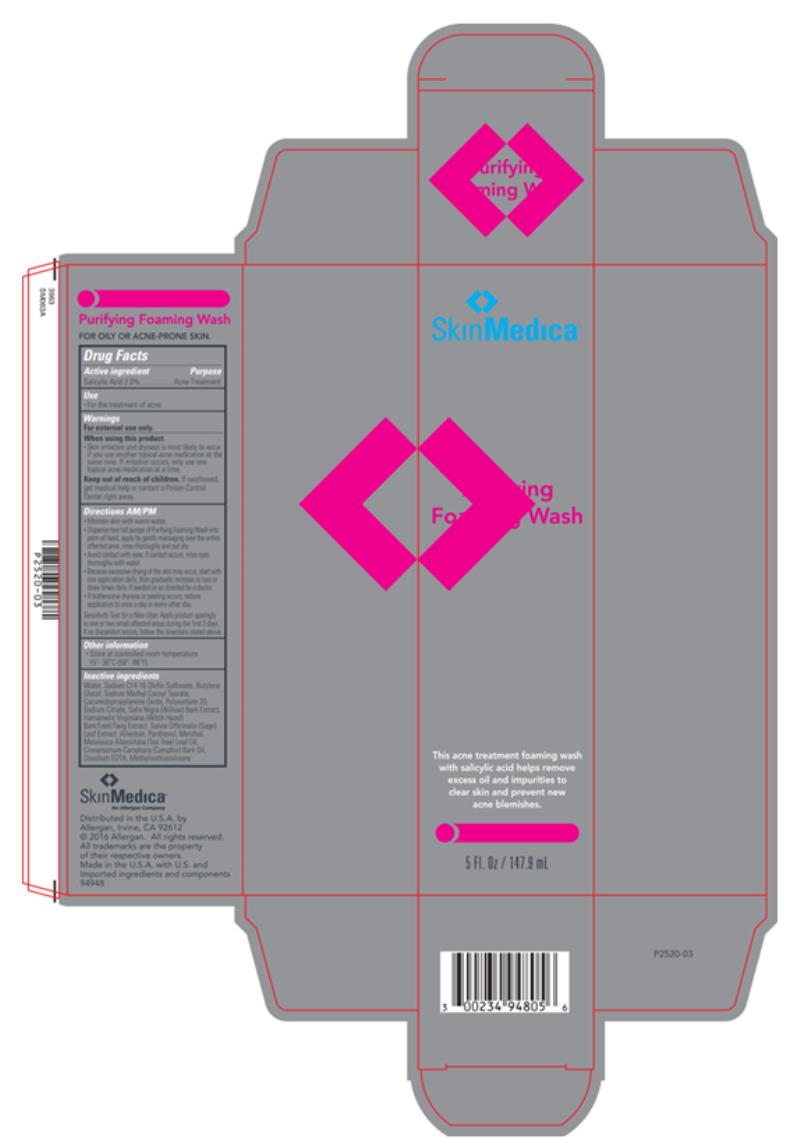
Label: SKIN MEDICA PURIFYING FOAMING WASH- salicylic acid liquid
- NDC Code(s): 0023-4948-05
- Packager: Allergan, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 1, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Keep out of reach of children.
-
Directions AM/PM
- Moisten skin with warm water.
- Dispense two full pumps of Purifying Foaming Wash into palm of hand, apply by gently massaging over the entire affected area, rinse thoroughly and pat dry.
- Avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Sensitivity Test for a New User. Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated above.
- Moisten skin with warm water.
- Other information
-
Inactive ingredients
Water, Sodium C14-16 Olefin Sulfonate, Butylene Glycol, Sodium Methyl Cocoyl Taurate, Cocamidopropylamine Oxide, Polysorbate 20, Sodium Citrate, Salix Nigra (Willow) Bark Extract, Hamamelis Virginiana (Witch Hazel) Bark/Leaf/Twig Extract, Salvia Officinalis (Sage) Leaf Extract, Allantoin, Panthenol, Menthol, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Cinnamomum Camphora (Camphor) Bark Oil, Disodium EDTA, Methylisothiazolinone
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SKIN MEDICA PURIFYING FOAMING WASH
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0023-4948 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength salicylic acid (UNII: O414PZ4LPZ) (salicylic acid - UNII:O414PZ4LPZ) salicylic acid 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAMPHOR OIL (UNII: 75IZZ8Y727) COCAMIDOPROPYLAMINE OXIDE (UNII: M4SL82J7HK) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) HAMAMELIS VIRGINIANA TOP (UNII: UDA30A2JJY) TEA TREE OIL (UNII: VIF565UC2G) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) PANTHENOL (UNII: WV9CM0O67Z) SALIX NIGRA BARK (UNII: QU52J3A5B3) SAGE (UNII: 065C5D077J) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) SODIUM METHYL COCOYL TAURATE (UNII: JVL98CG53G) POLYSORBATE 20 (UNII: 7T1F30V5YH) WATER (UNII: 059QF0KO0R) SODIUM CITRATE (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0023-4948-05 1 in 1 CARTON 01/01/2013 1 147.9 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 01/01/2013 Labeler - Allergan, Inc. (144796497)