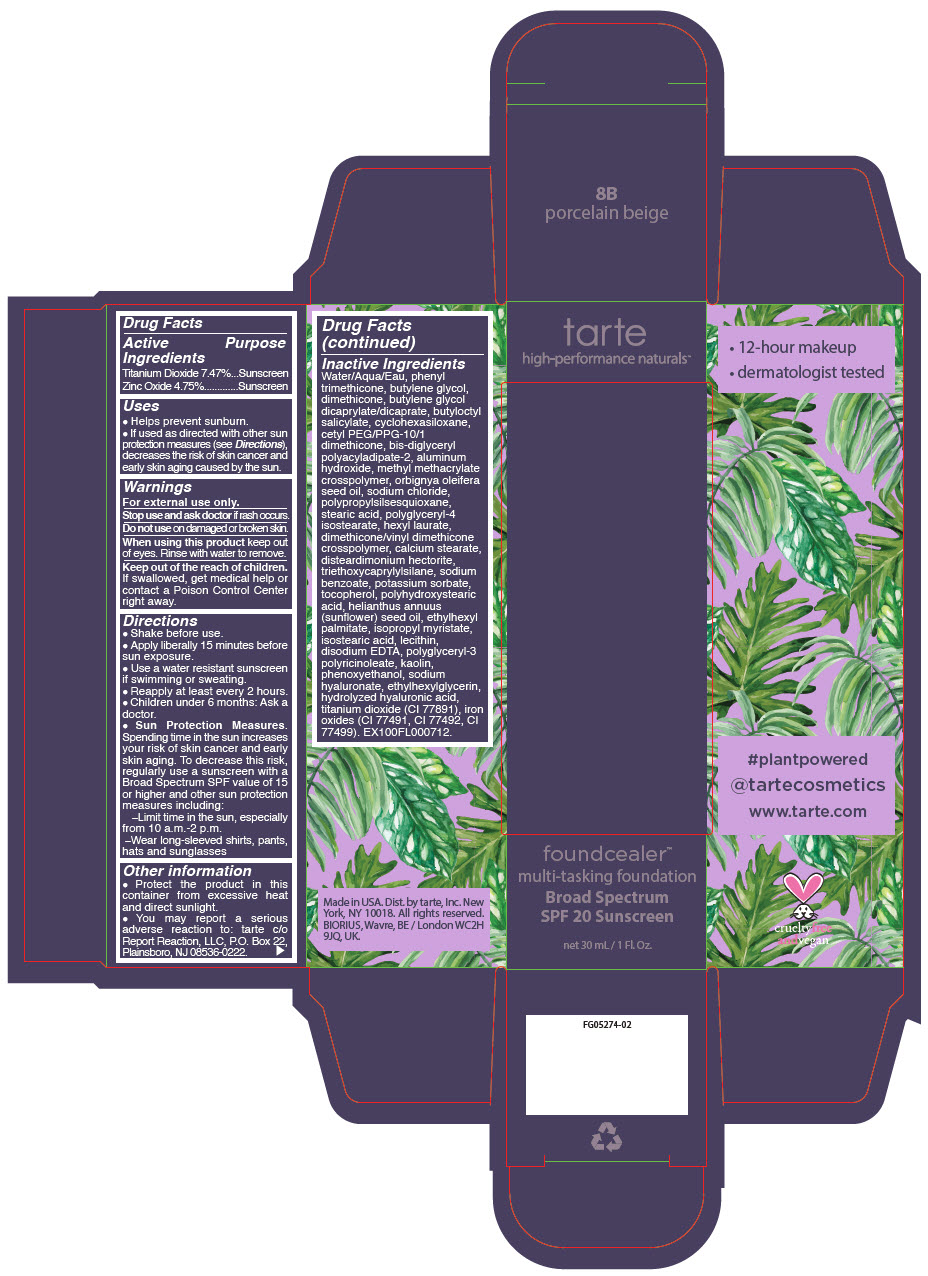
Label: FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 8B PORCELAIN BEIGE- titanium dioxide and zinc oxide liquid
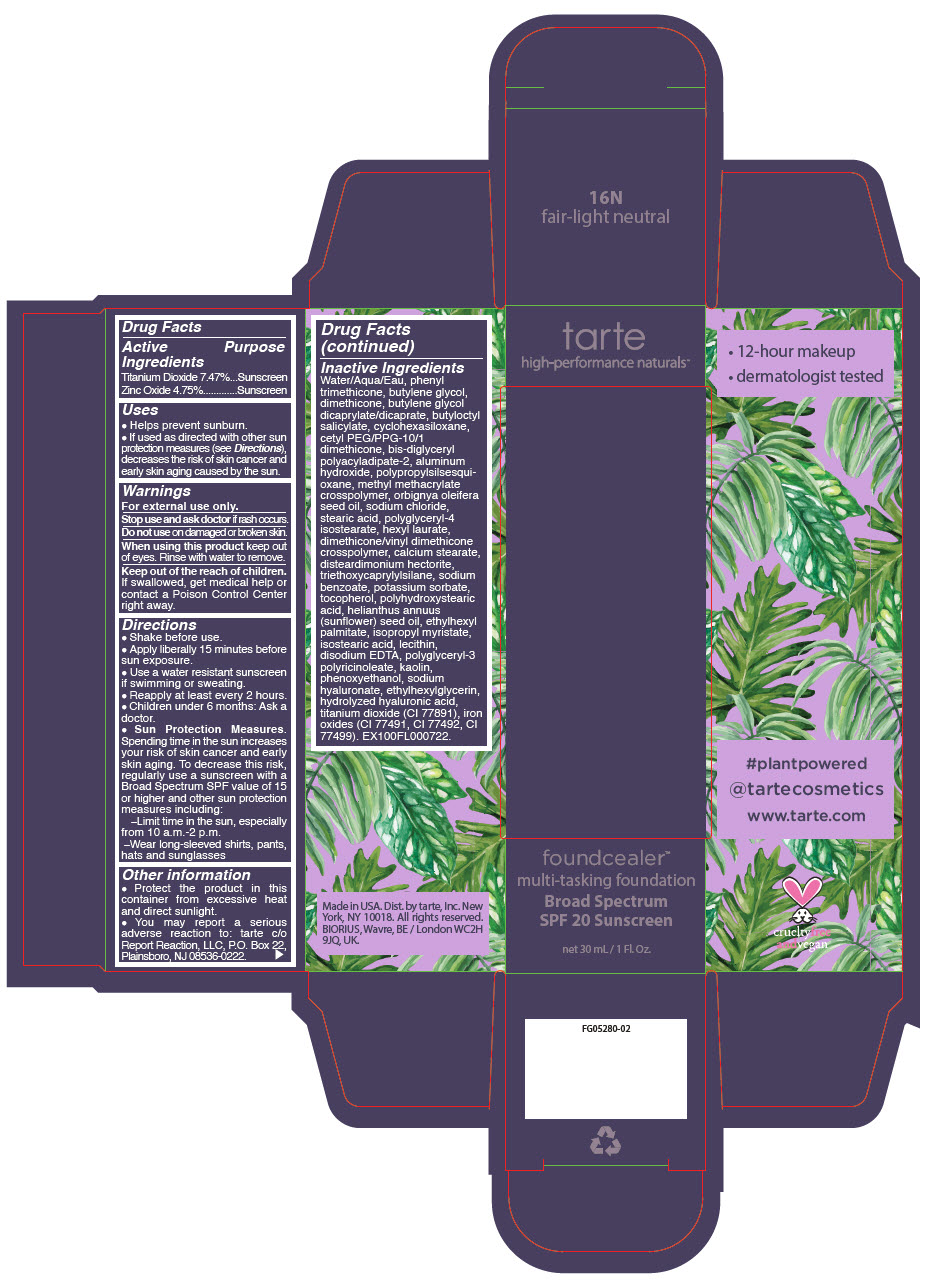
FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 13N FAIR NEUTRAL- titanium dioxide and zinc oxide liquid
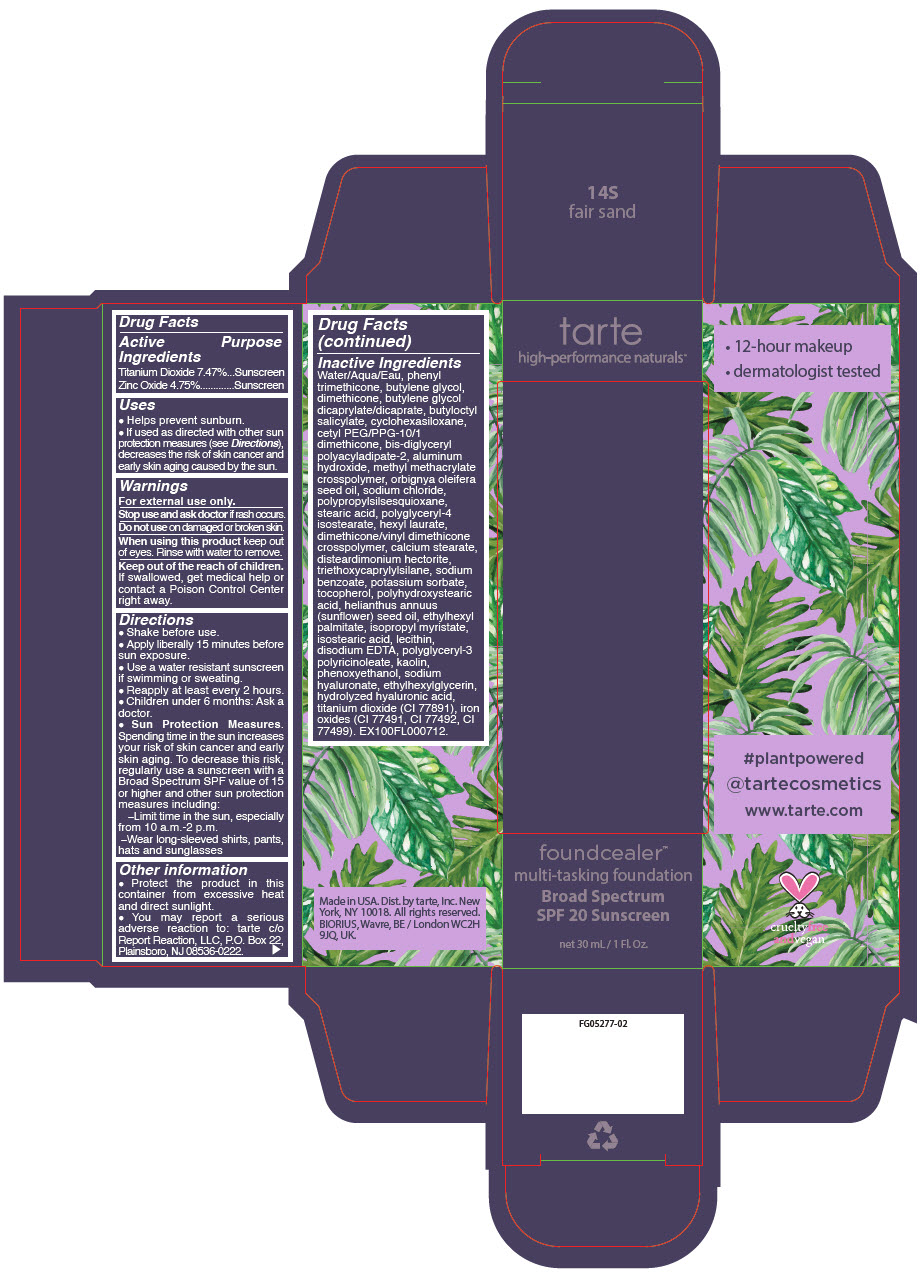
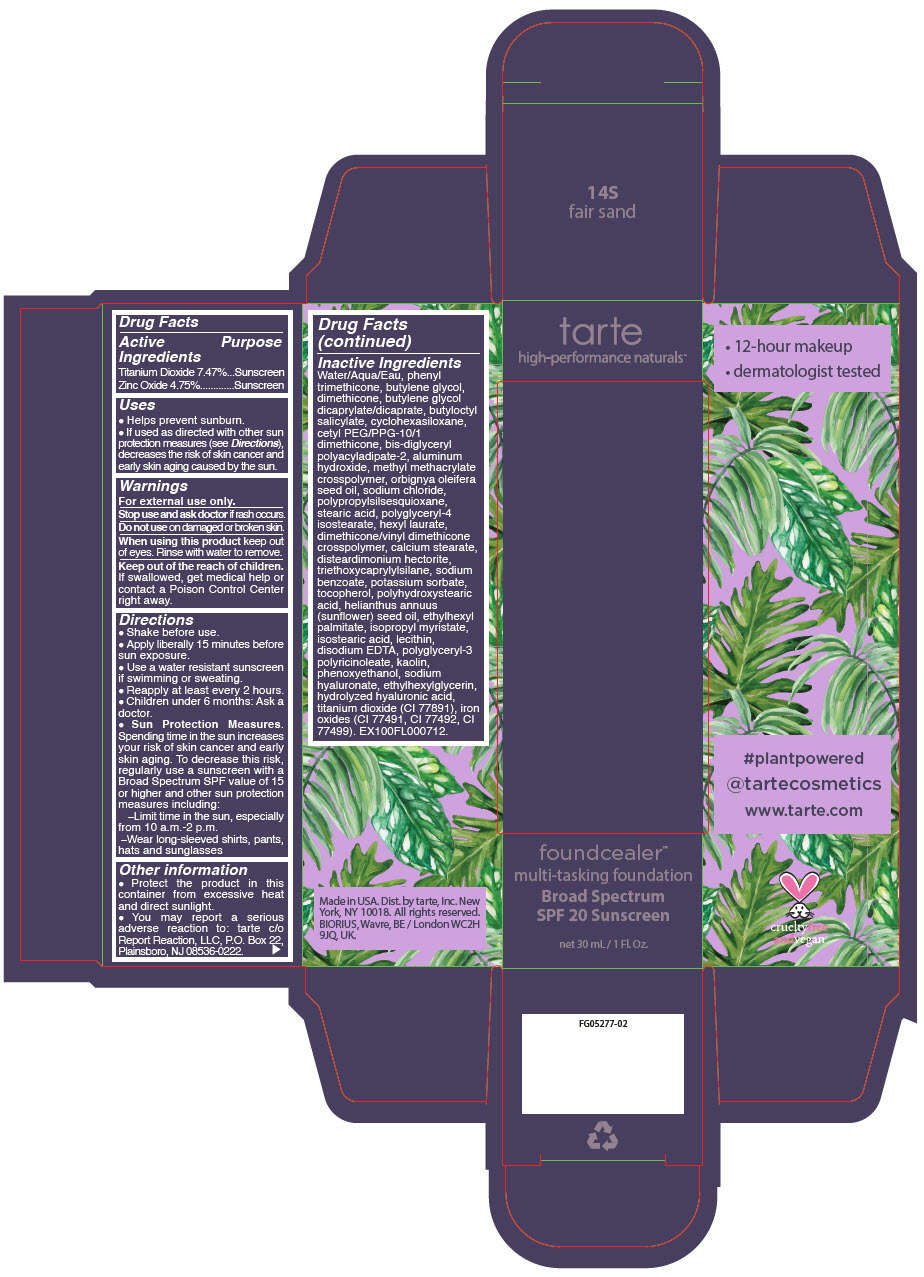
FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 14S FAIR SAND (titanium dioxide and zinc oxide) l ......./strong> FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 58S RICH SAND (titanium dioxide and zinc oxide) liquid FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 59N RICH NEUTRAL (titanium dioxide and zinc oxide) liquid FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 60H MAHOGANY- titanium dioxide and zinc oxide liquid [Tarte, Inc.]
FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 58S RICH SAND- titanium dioxide and zinc oxide liquid
FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 59N RICH NEUTRAL- titanium dioxide and zinc oxide liquid
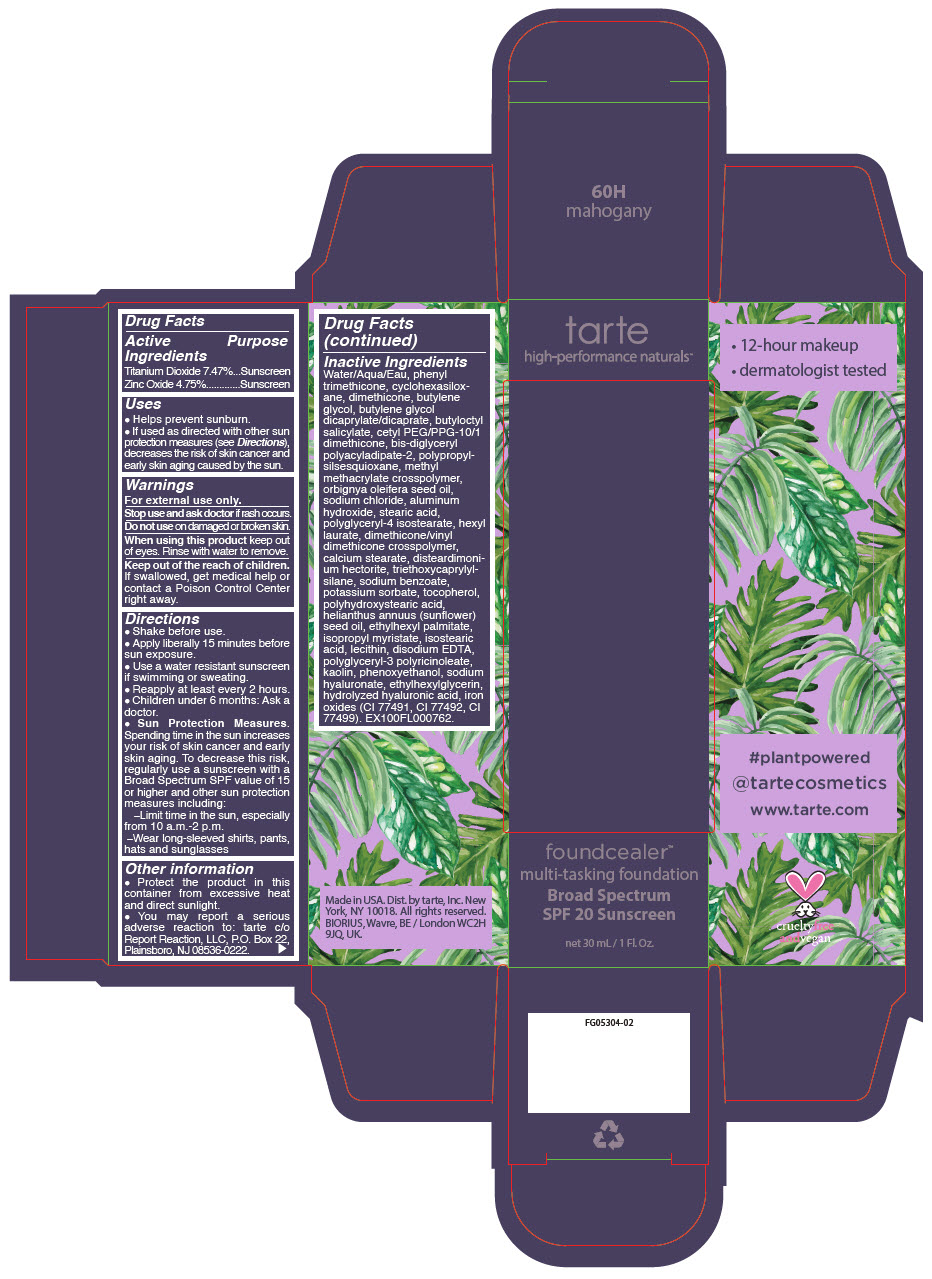
FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 60H MAHOGANY- titanium dioxide and zinc oxide liquid
-
NDC Code(s):
51060-197-01,
51060-198-01,
51060-199-01,
51060-200-01, view more51060-201-01, 51060-202-01, 51060-203-01, 51060-204-01, 51060-205-01, 51060-206-01, 51060-207-01, 51060-208-01, 51060-209-01, 51060-210-01, 51060-211-01, 51060-212-01, 51060-213-01, 51060-214-01, 51060-215-01, 51060-216-01, 51060-217-01, 51060-218-01, 51060-219-01, 51060-220-01, 51060-221-01, 51060-222-01, 51060-223-01, 51060-224-01, 51060-225-01, 51060-226-01
- Packager: Tarte, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Shake before use.
- Apply liberally 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Children under 6 months: Ask a doctor.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- –
- Limit time in the sun, especially from 10 a.m.-2 p.m.
- –
- Wear long-sleeved shirts, pants, hats and sunglasses
- Other information
-
Inactive Ingredients
Water/Aqua/Eau, phenyl trimethicone, butylene glycol, dimethicone, butylene glycol dicaprylate/dicaprate, butyloctyl salicylate, cyclohexasiloxane, cetyl PEG/PPG-10/1 dimethicone, bis-diglyceryl polyacyladipate-2, aluminum hydroxide, methyl methacrylate crosspolymer, orbignya oleifera seed oil, sodium chloride, polypropylsilsesquioxane, stearic acid, polyglyceryl-4 isostearate, hexyl laurate, dimethicone/vinyl dimethicone crosspolymer, calcium stearate, disteardimonium hectorite, triethoxycaprylylsilane, sodium benzoate, potassium sorbate, tocopherol, polyhydroxystearic acid, helianthus annuus (sunflower) seed oil, ethylhexyl palmitate, isopropyl myristate, isostearic acid, lecithin, disodium EDTA, polyglyceryl-3 polyricinoleate, kaolin, phenoxyethanol, sodium hyaluronate, ethylhexylglycerin, hydrolyzed hyaluronic acid, titanium dioxide (CI 77891), iron oxides (CI 77491, CI 77492, CI 77499). EX100FL000712.
- SPL UNCLASSIFIED SECTION
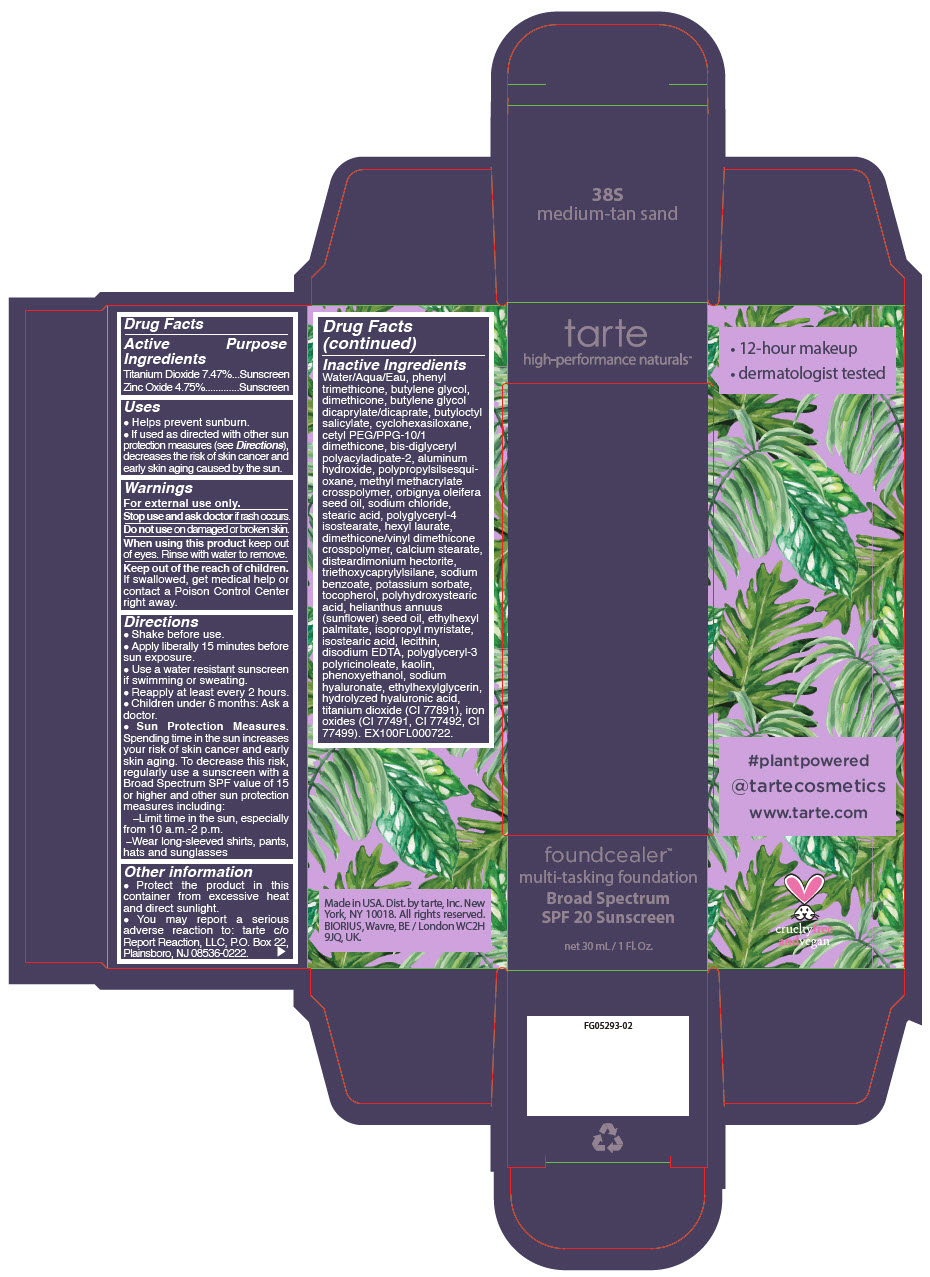
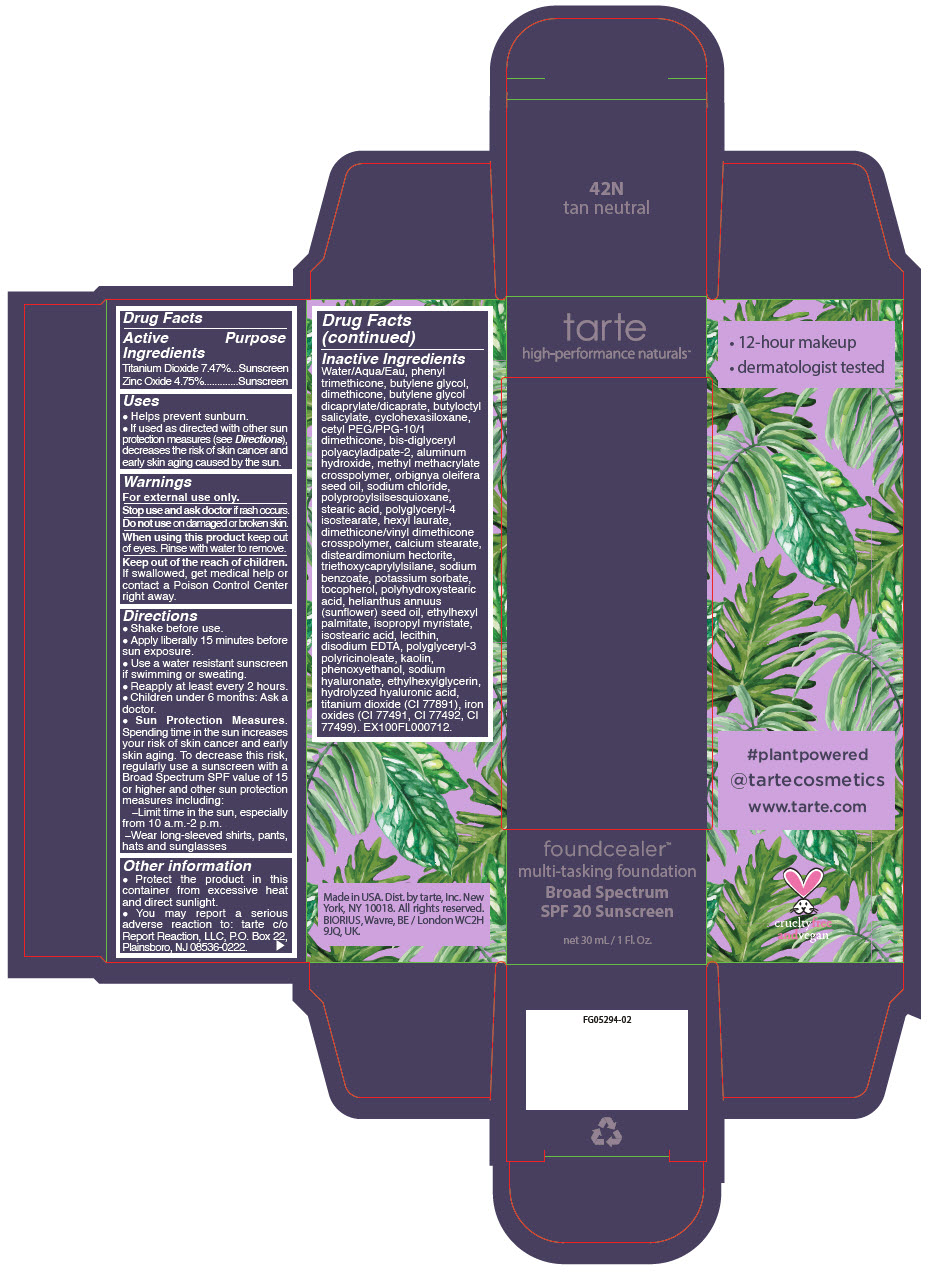
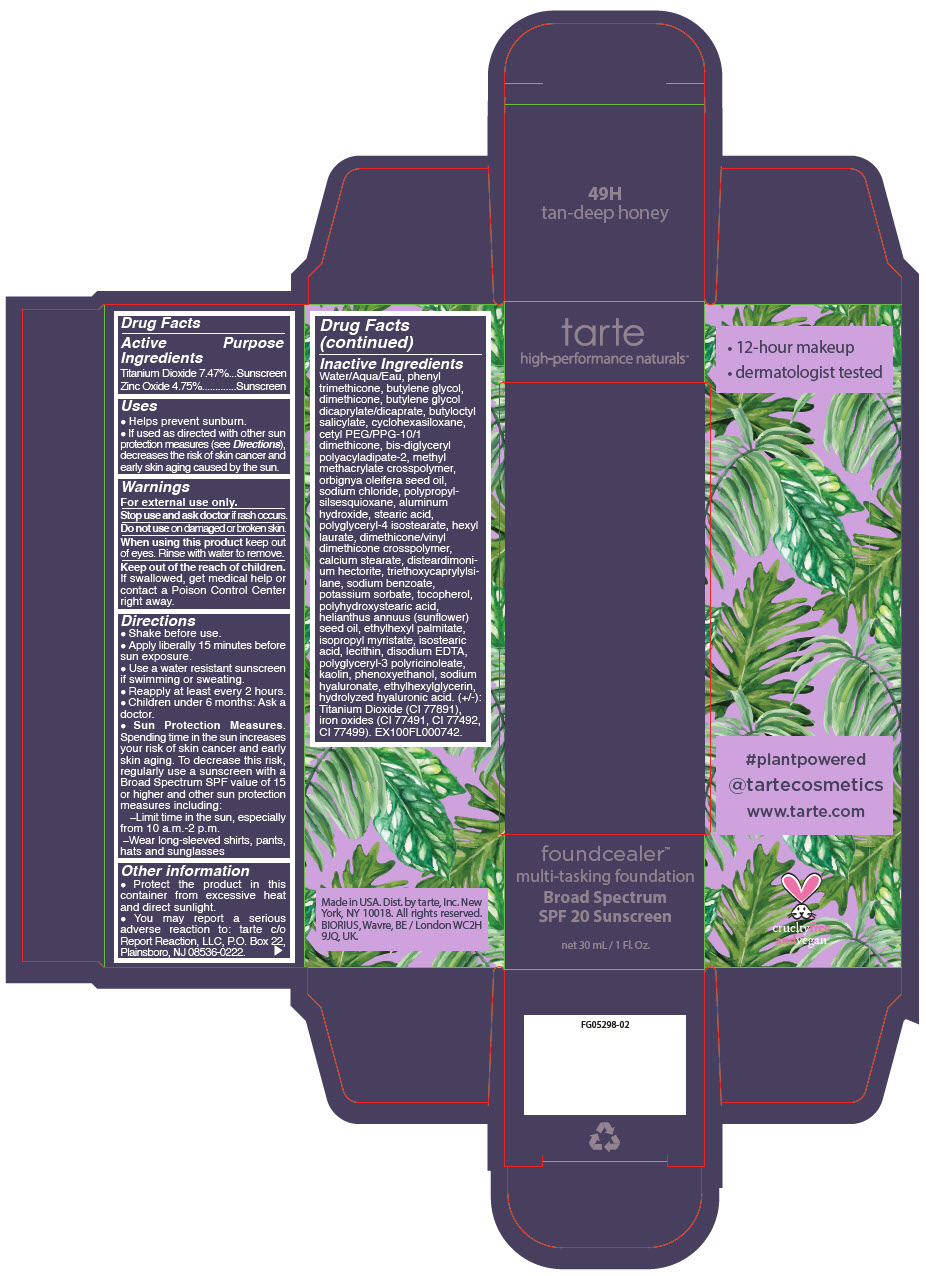
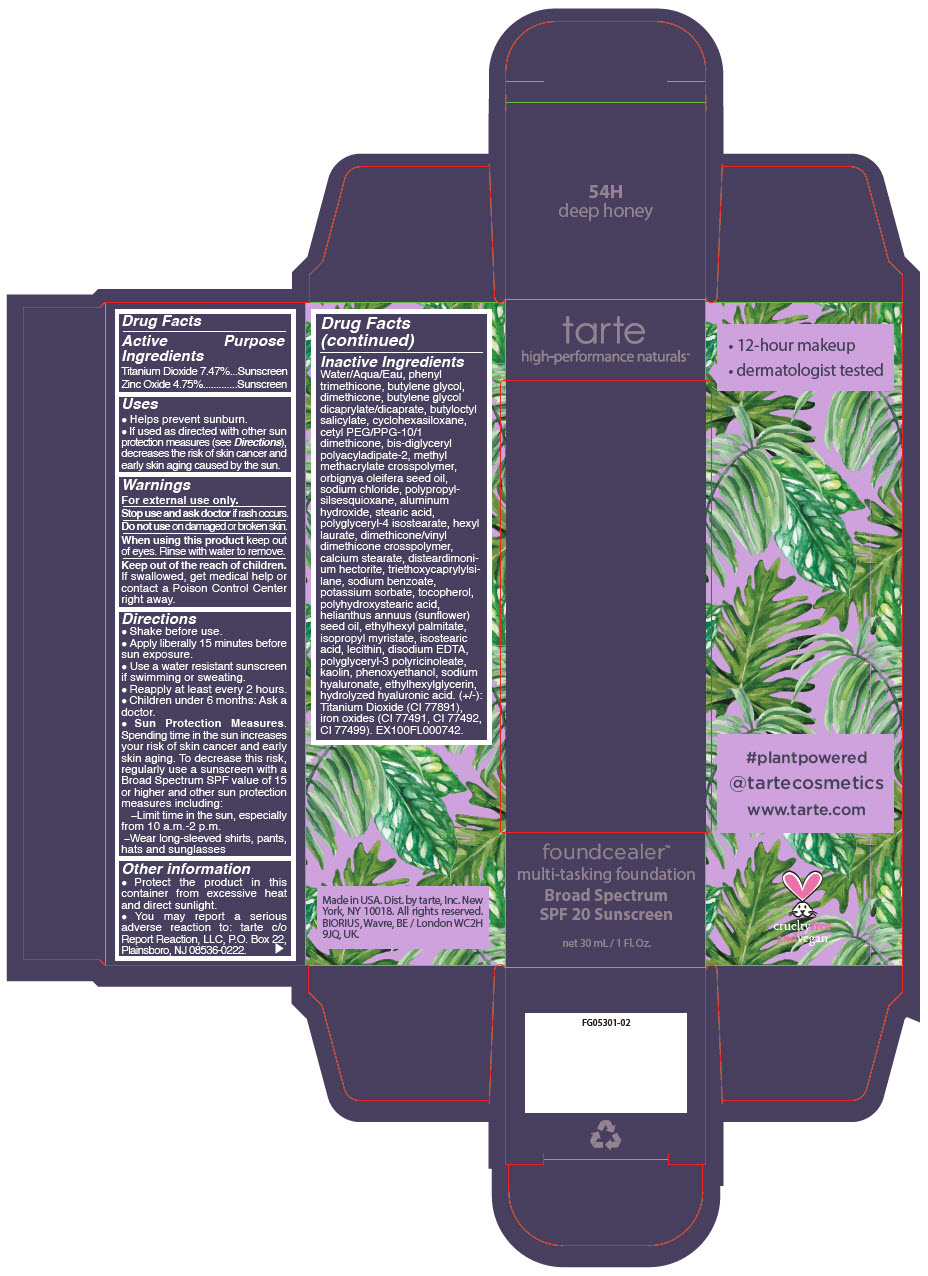
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 8B - porcelain beige
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 13N - fair neutral
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 14S - fair sand
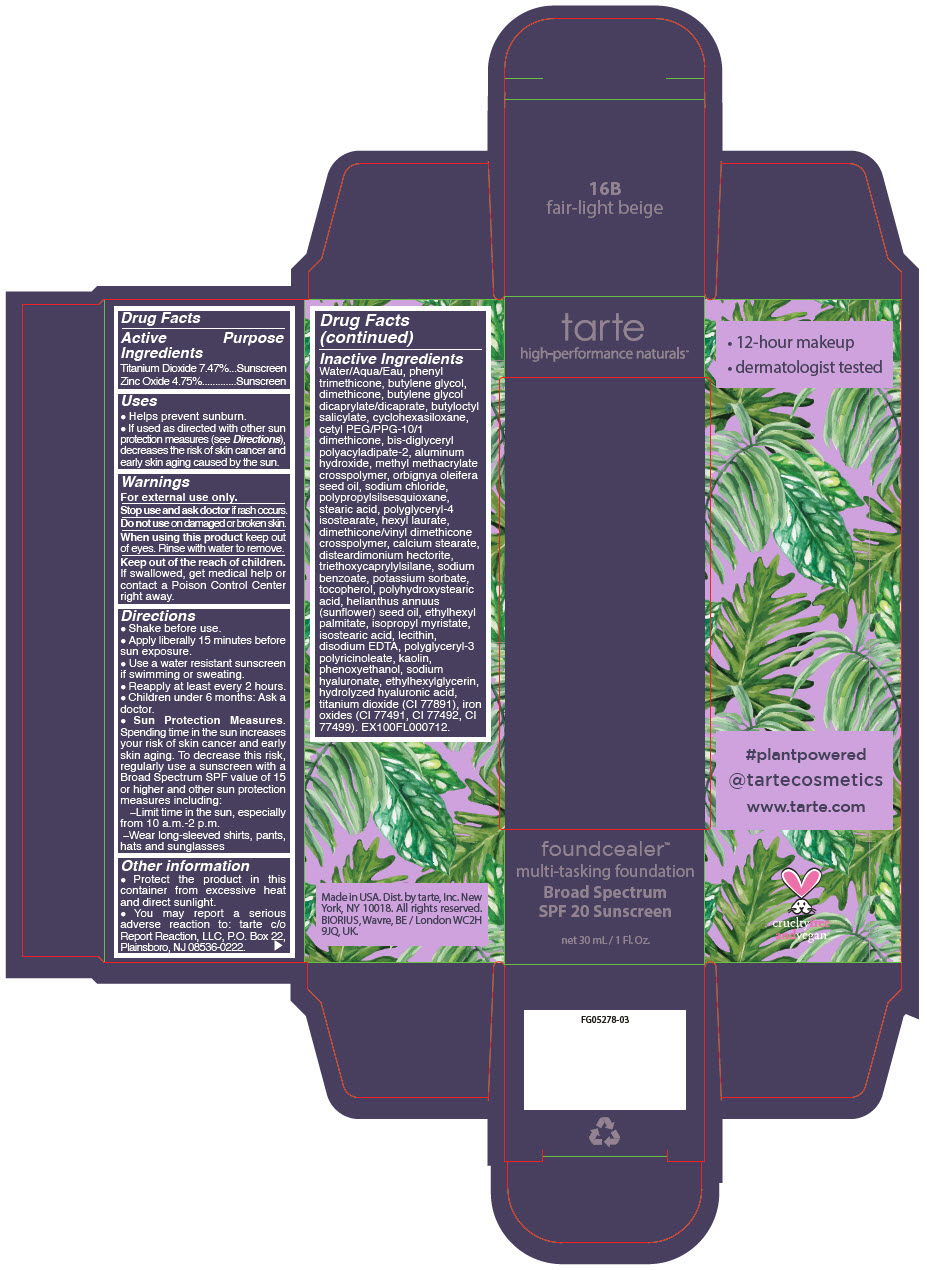
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 16B - fair-light beige
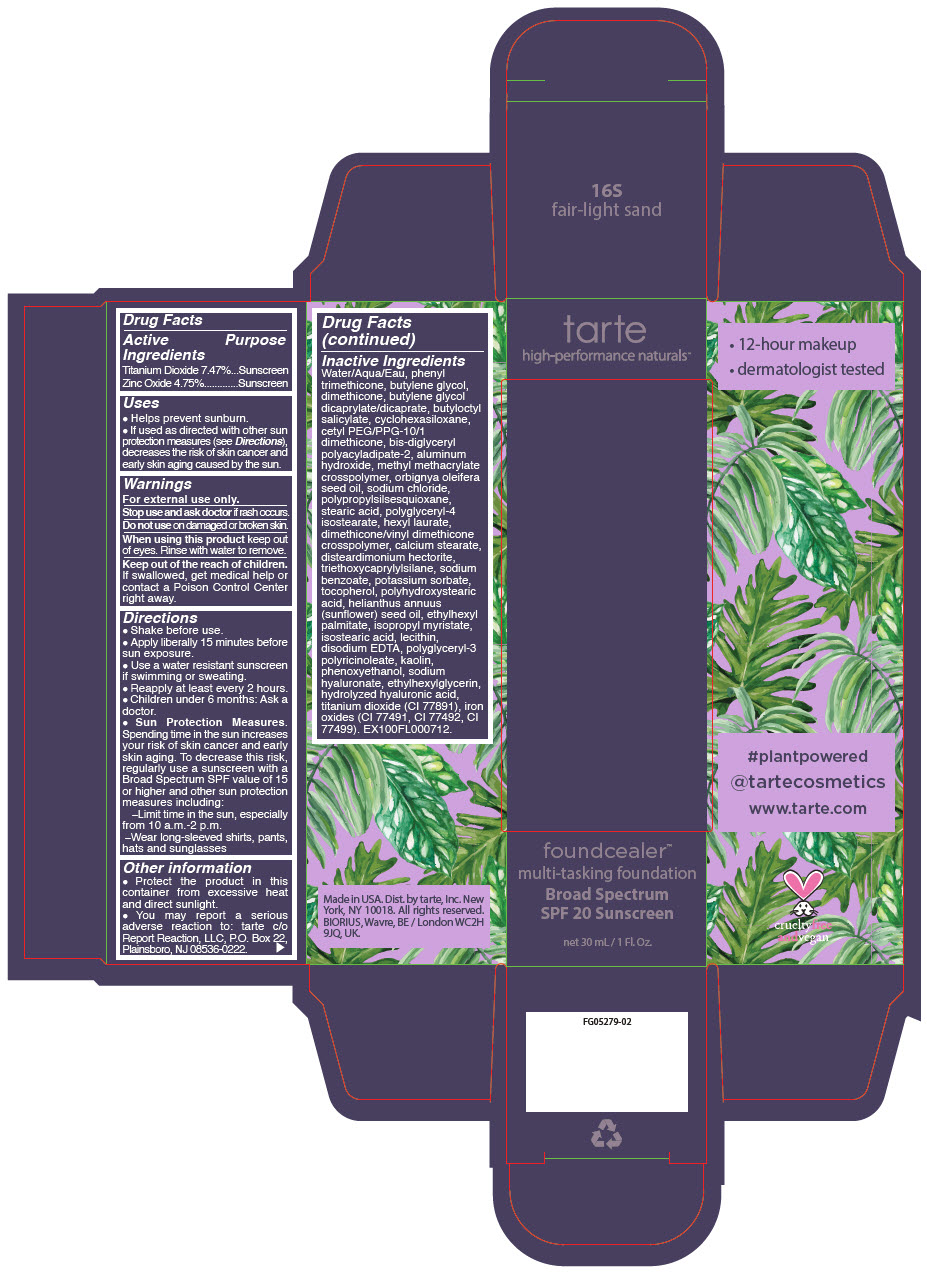
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 16S - fair-light sand
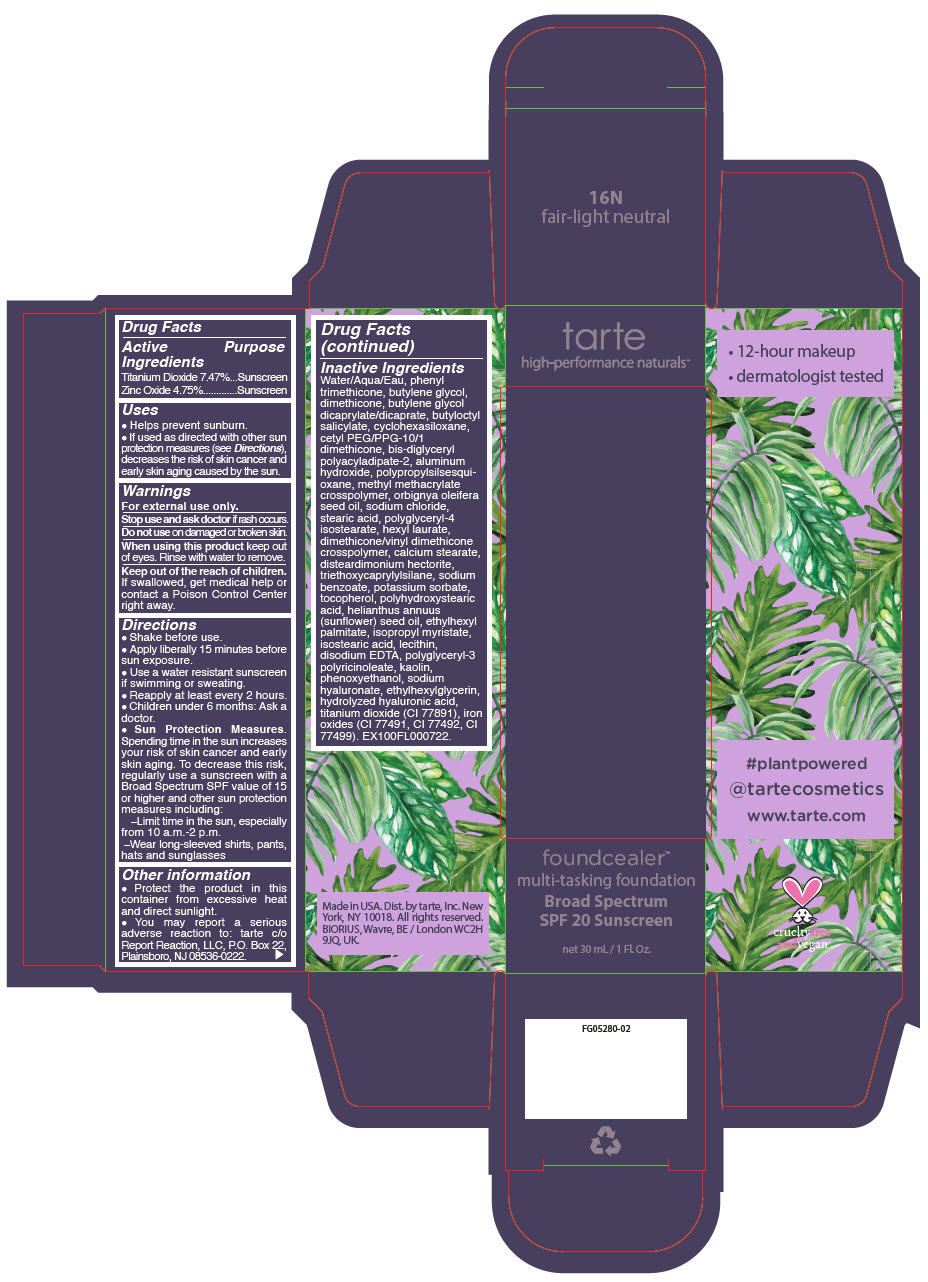
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 16N - fair-light neutral
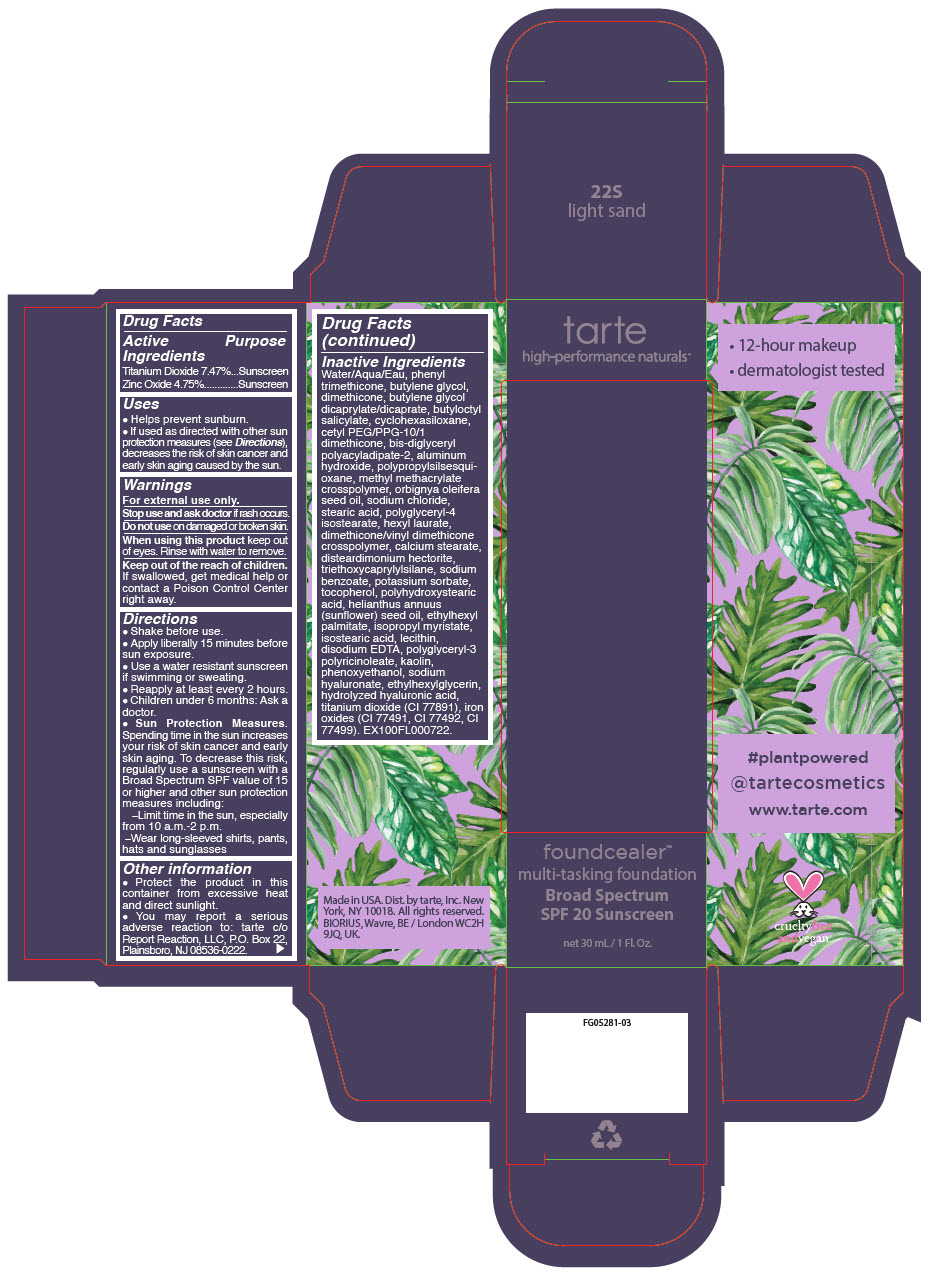
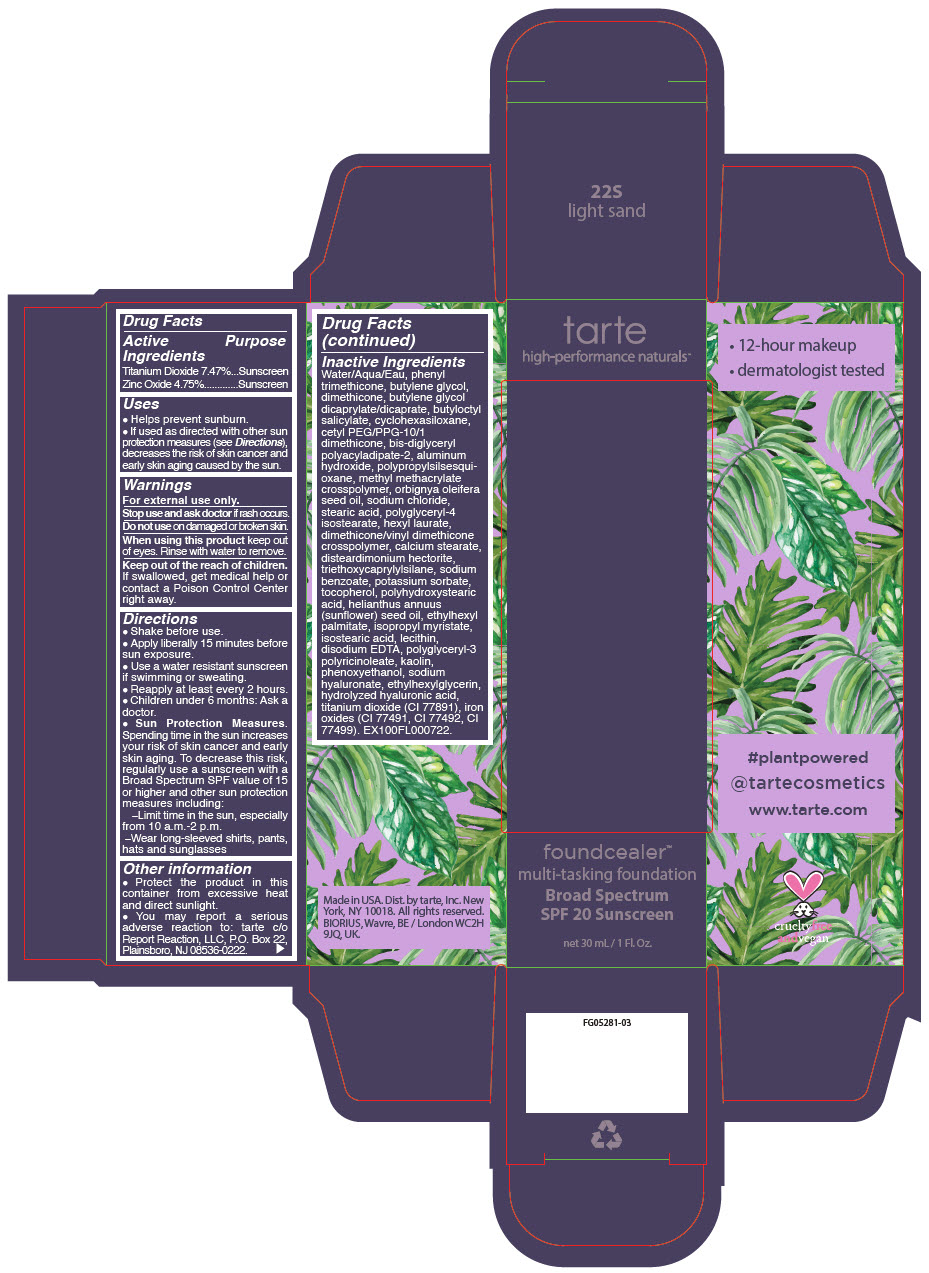
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 22S - light sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 22N - light neutral
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 22B - light beige
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 24G - light golden
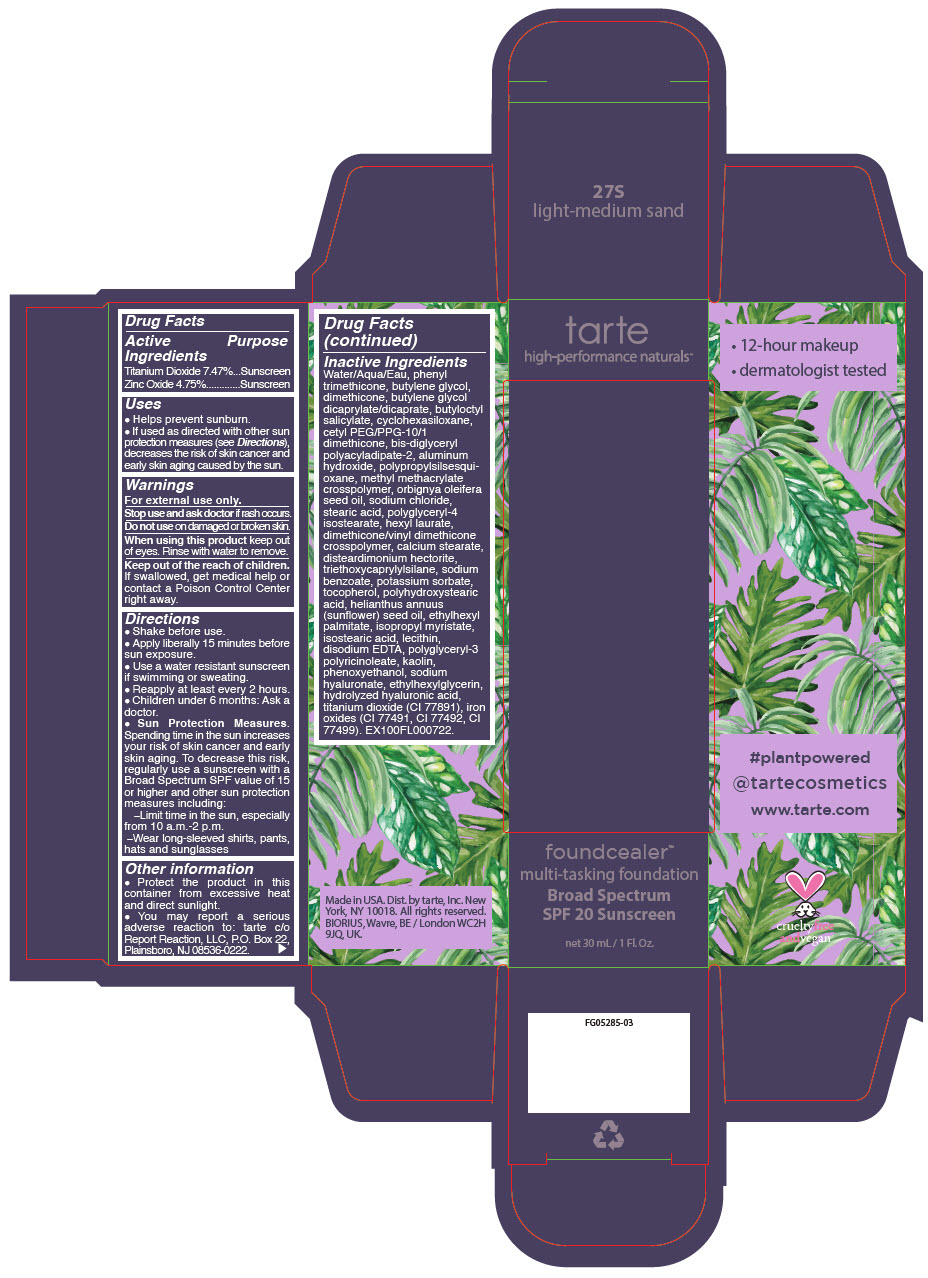
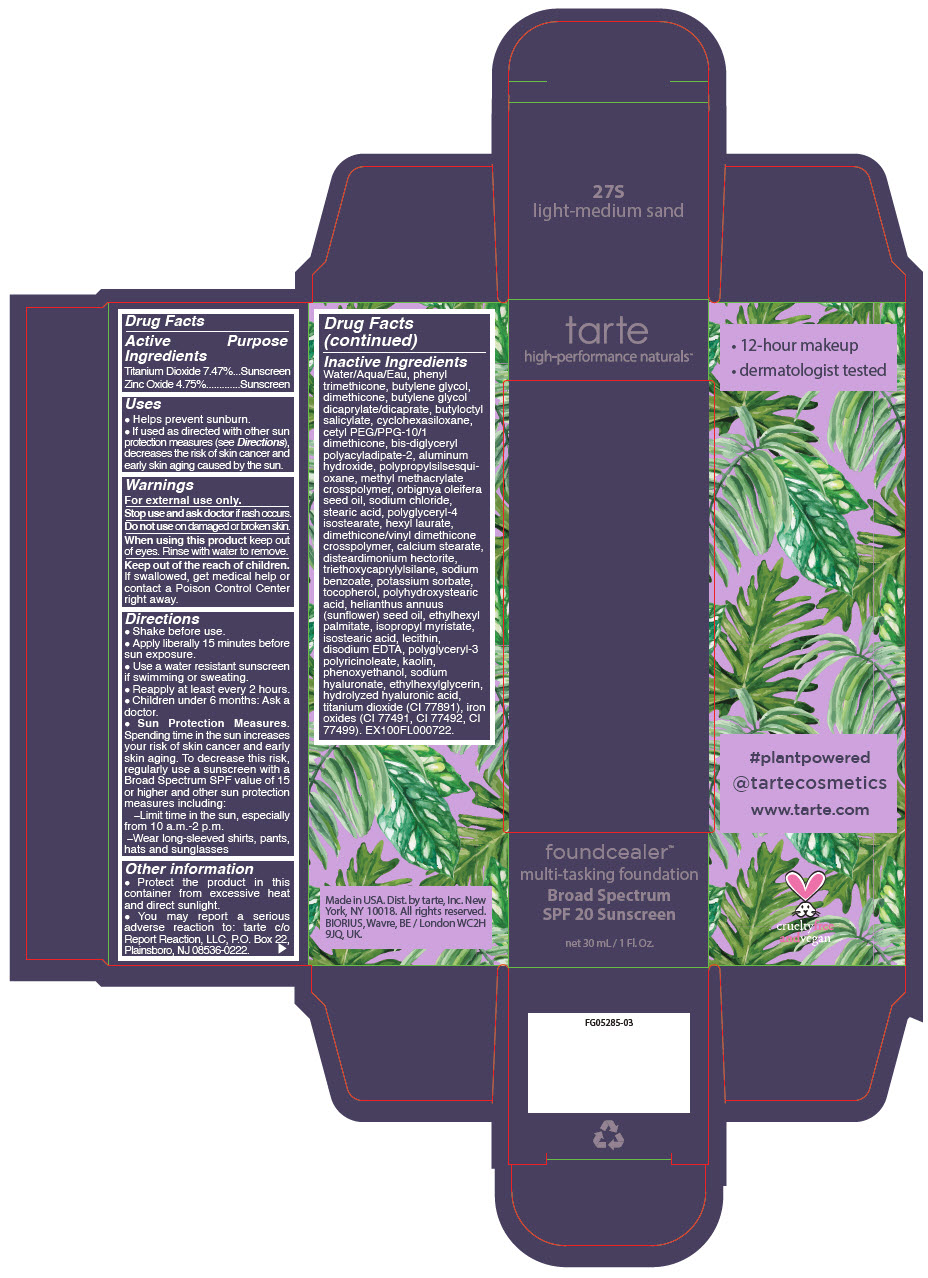
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 27S - light-medium sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 27N - light-medium neutral
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 29H - light-medium honey
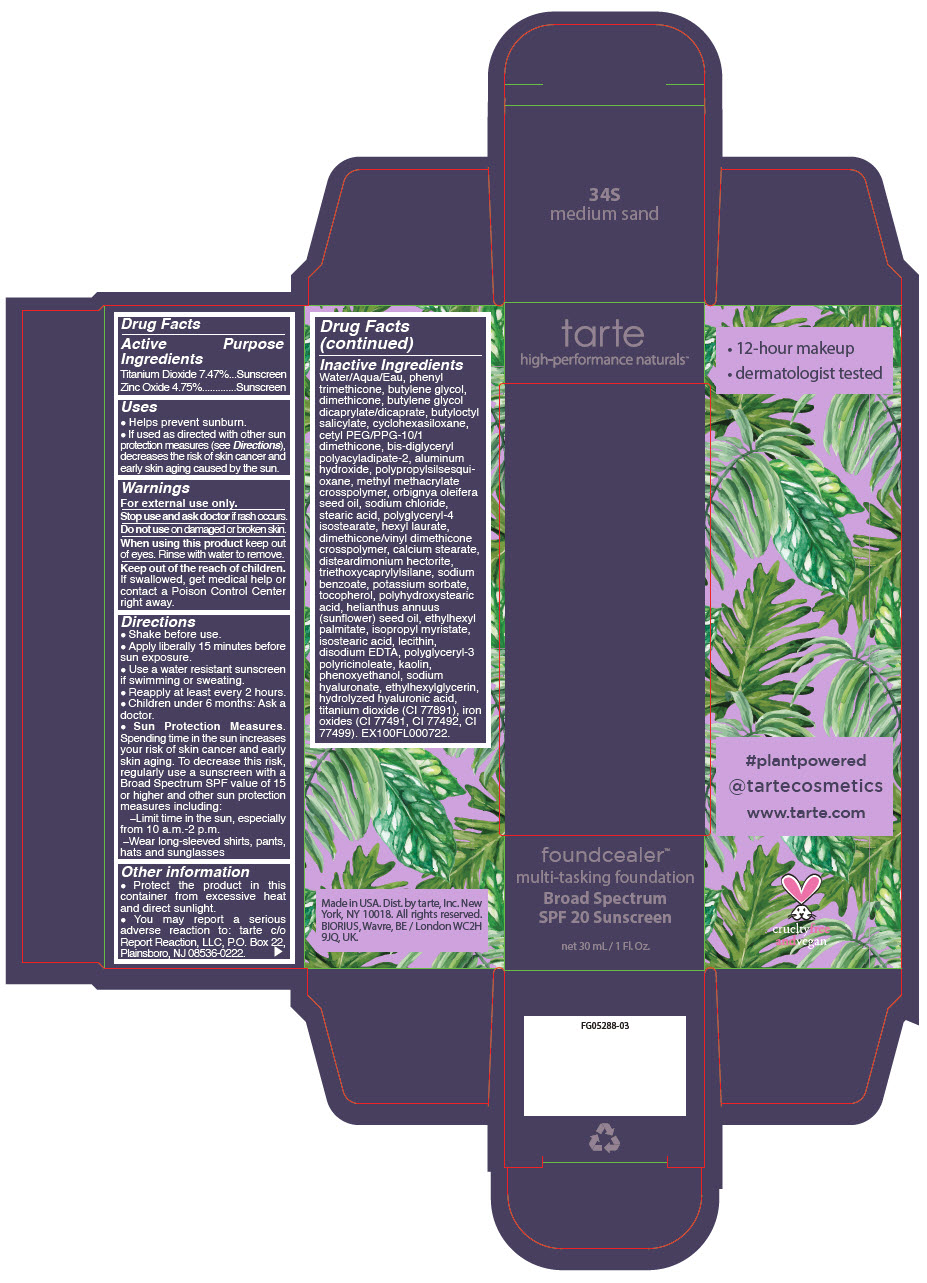
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 34S - medium sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 34N - medium neutral
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 35G - medium golden
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 36H - medium-tan honey
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 36N - medium-tan neutral
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 38S - medium-tan sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 42N - tan neutral
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 44H - tan honey
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 44S - tan sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 48S - tan-deep sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 49H - tan-deep honey
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 50S - deep sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 52N - deep neutral
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 54H - deep honey
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 58S - rich sand
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 59N - rich neutral
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 60H - mahogany
-
INGREDIENTS AND APPEARANCE
FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 8B PORCELAIN BEIGE
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-197 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-197-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 13N FAIR NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-198 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-198-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 14S FAIR SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-199 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-199-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 16B FAIR-LIGHT BEIGE
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-200 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-200-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 16S FAIR-LIGHT SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-201 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-201-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 16N FAIR-LIGHT NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-202 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-202-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 22S LIGHT SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-203 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-203-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 22N LIGHT NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-204 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-204-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 22B LIGHT BEIGE
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-205 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-205-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 24G LIGHT GOLDEN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-206 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-206-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 27S LIGHT-MEDIUM SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-207 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-207-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 27N LIGHT-MEDIUM NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-208 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-208-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 29H LIGHT-MEDIUM HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-209 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-209-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 34S MEDIUM SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-210 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-210-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 34N MEDIUM NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-211 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-211-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 35G MEDIUM GOLDEN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-212 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-212-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 36H MEDIUM-TAN HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-213 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-213-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 36N MEDIUM-TAN NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-214 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-214-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 38S MEDIUM-TAN SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-215 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-215-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 42N TAN NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-216 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-216-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 44H TAN HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-217 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-217-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 44S TAN SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-218 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-218-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 48S TAN-DEEP SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-219 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-219-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 49H TAN-DEEP HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-220 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-220-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 50S DEEP SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-221 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-221-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 52N DEEP NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-222 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-222-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 54H DEEP HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-223 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CALCIUM STEARATE (UNII: 776XM7047L) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-223-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 58S RICH SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-224 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) CALCIUM STEARATE (UNII: 776XM7047L) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-224-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 59N RICH NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-225 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) CALCIUM STEARATE (UNII: 776XM7047L) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-225-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 FOUNDCEALER MULTI-TASKING FOUNDATION BROAD SPECTRUM SPF 20 SUNSCREEN 60H MAHOGANY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-226 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 74.7 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 47.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) BABASSU OIL (UNII: 8QSB4M5477) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) CALCIUM STEARATE (UNII: 776XM7047L) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) KAOLIN (UNII: 24H4NWX5CO) CYCLOMETHICONE 4 (UNII: CZ227117JE) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONIC ACID (UNII: S270N0TRQY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-226-01 1 in 1 CARTON 03/21/2019 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2019 Labeler - Tarte, Inc. (027905186)