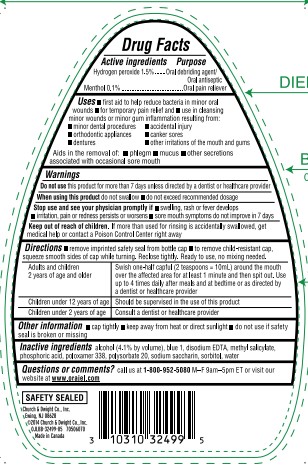
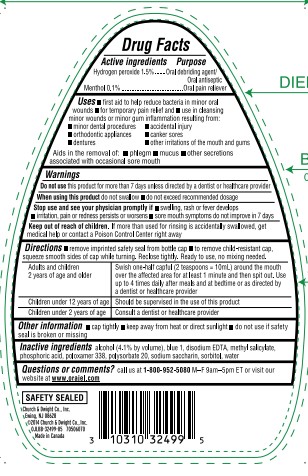
Label: ORAJEL ANTISEPTIC RINSE FOR ALL MOUTH SORES- hydrogen peroxide and menthol liquid
- NDC Code(s): 10237-760-16, 10237-760-48
- Packager: Church & Dwight Co., Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
-
INDICATIONS & USAGE
- first aid to help reduce bacteria in minor oral wounds
- for temporary pain relief and
- use in cleansing minor wounds or minor gum inflammation resulting from:
- minor dental procedures
- accidental injury
- orthodontic appliances
- canker sores
- dentures
- other irritations of the mouth and gums
Aids in the removal of:
- phlegm
- mucus
- other secretions associated with occasional sore mouth
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
- remove imprinted safety seal from bottle cap
- to remove child-resistant cap, squeeze smooth sides of cap while turning. Reclose tightly. Ready to use, no mixing needed.
Adults and children
2 years of age and older
Swish one-half capful (2 tesapoons=10mL) around the mouth over the affected area for at least 1 minute and then spit out. Use up to 4 times daily after meals and at bedtime or as directed by a dentist or healthcare provider Children under 12 years of age Should be supervised in the use of this product Children under 2 years of age Consult a dentist or healthcare provider - OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
-
QUESTIONS
Questions or comments call us at 1–800–952–5080 M–F 9am–5pm ET or visit our website atwww.orajel.com
-
PRINCIPAL DISPLAY PANEL
#1
ORAL PAIN
RELIEVER BRAND
FOR ADULTS
With
PAIN RELIEF!
Orajel™
FOR ALL MOUTH SORES
ANTISEPTIC RINSE
Canker Sores Cheek Bites Gum Irritation
Irritation from Dentures or Braces
Promotes Healing
Kills Bacteria
Provides Pain Relief
SOOTHING
MINT
RINSE
ORAL DEBRIDING AGENT / ANTISEPTIC RINSE / PAIN RELIEVER
16 FL OZ (473.2 mL)

-
INGREDIENTS AND APPEARANCE
ORAJEL ANTISEPTIC RINSE FOR ALL MOUTH SORES
hydrogen peroxide and menthol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10237-760 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROGEN PEROXIDE (UNII: BBX060AN9V) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) HYDROGEN PEROXIDE 15 mg in 1 mL MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) EDETATE DISODIUM (UNII: 7FLD91C86K) METHYL SALICYLATE (UNII: LAV5U5022Y) PHOSPHORIC ACID (UNII: E4GA8884NN) POLOXAMER 338 (UNII: F75JV2T505) POLYSORBATE 20 (UNII: 7T1F30V5YH) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10237-760-16 473.2 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2014 2 NDC:10237-760-48 1419.5 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part356 11/01/2014 Labeler - Church & Dwight Co., Inc. (001211952) Establishment Name Address ID/FEI Business Operations Church & Dwight Canada Corp. 253933600 MANUFACTURE(10237-760)