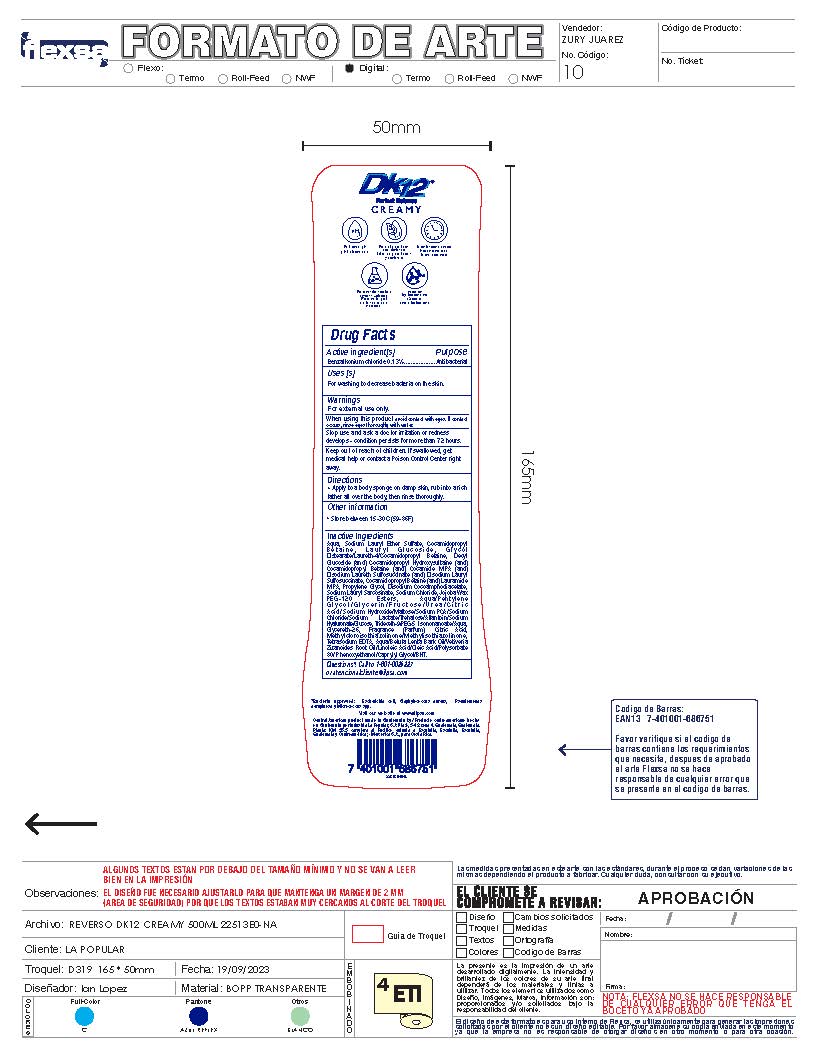
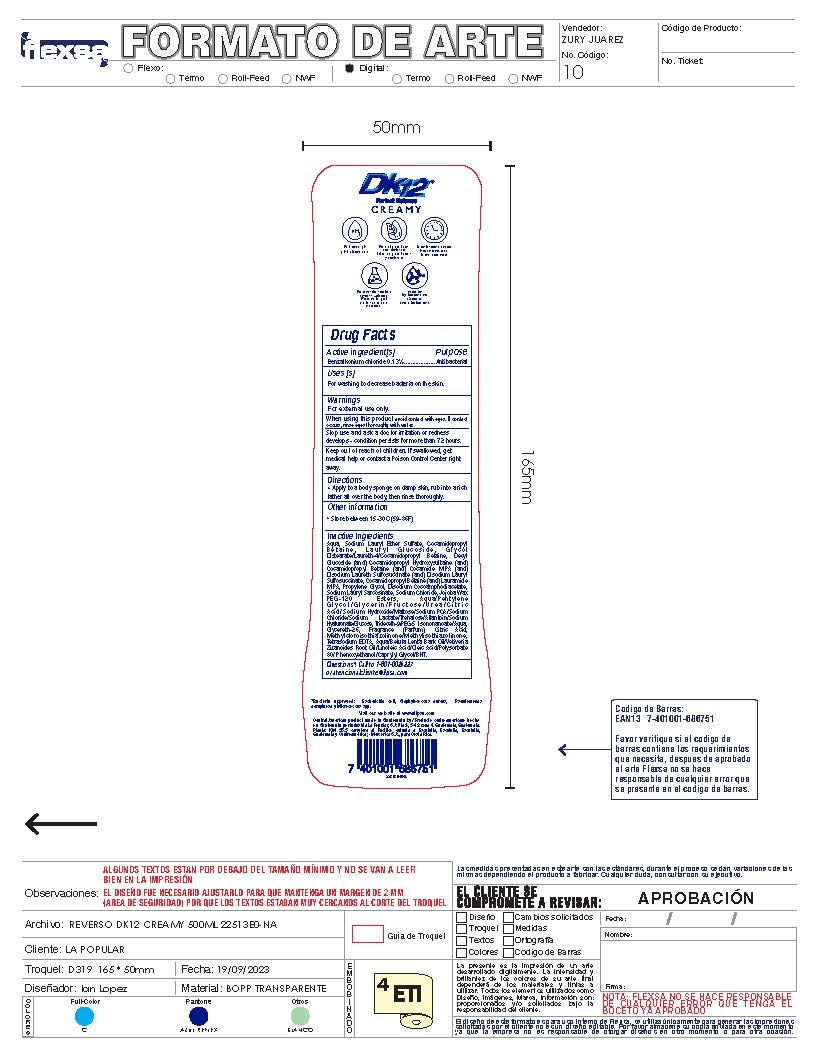
Label: DK12 SHOWER GEL CREAMY- benzalkonium chloride 0.13% gel
- NDC Code(s): 62476-010-16
- Packager: Industria La Popular, S.A.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other Information
-
Inactive Ingredients
Aqua, Sodium Lauryl Ether Sulfate, Cocamidopropyl B e t a i n e , L a u r y l G l u c o s i d e , G l y c o l
Distearate/Laureth-4/Cocamidopropyl Betaine, Decyl Glucoside (and) Cocamidopropyl Hydroxysultaine (and) Cocamidopropyl Betaine (and) Cocamide MIPA (and) Disodium Laureth Sulfosuccinate (and) Disodium Lauryl Sulfosuccinate, Cocamidopropyl Betaine (and) Lauramide MIPA, Propylene Glycol, Disodium Cocoamphodiacetate, Sodium Lauryl Sarcosinate, Sodium Chloride, Jojoba Wax PEG-120 Esters, Aqua/Pentylene G l y c o l / G l y c e r i n / F r u c t o s e / U r e a / C i t r i c Acid/Sodium Hydroxide/Maltose/Sodium PCA/Sodium Chloride/Sodium Lactate/Trehalose/Allantoin/Sodium Hyaluronate/Glucose, Trideceth-9/PEG-5 Isononanoate/Aqua, Glycereth-26, Fragrance (Parfum), Citric Acid, Methylcloroisothiazolinone/Methylisothiazolinone, Tetrasodium EDTA, Aqua/Betula Lenta Bark Oil/Vetiveria Zizanoides Root Oil/Linoleic Acid/Oleic Acid/Polysorbate 80/ Phenoxyethanol/Caprylyl Glycol/BHT. - DK12 Shower Gel Creamy
-
INGREDIENTS AND APPEARANCE
DK12 SHOWER GEL CREAMY
benzalkonium chloride 0.13% gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62476-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.65 g in 500 mL Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) MALTOSE (UNII: XJ6S9RV06F) GLYCERETH-26 (UNII: NNE56F2N14) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) LAURAMIDE (UNII: 3BD22052MO) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) COCAMIDOPROPYL HYDROXYSULTAINE (UNII: 62V75NI93W) HYDROLYZED JOJOBA ESTERS (ACID FORM) (UNII: UDR641JW8W) DISODIUM LAURYL SULFOSUCCINATE (UNII: P160Q81342) SODIUM LAURYL SARCOSINATE (UNII: 5PGH842FAU) SODIUM CHLORIDE (UNII: 451W47IQ8X) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) UREA (UNII: 8W8T17847W) TREHALOSE (UNII: B8WCK70T7I) LAURETH-4 (UNII: 6HQ855798J) COCAMIDE (UNII: 3YXD33R71G) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) SODIUM LAURYL SULFATE (UNII: 368GB5141J) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCOL DISTEARATE (UNII: 13W7MDN21W) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) EDETATE SODIUM (UNII: MP1J8420LU) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DISODIUM COCOAMPHODIACETATE (UNII: 18L9G3U51M) BETULA LENTA BARK (UNII: J689T0DVJQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PENTYLENE GLYCOL (UNII: 50C1307PZG) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM LACTATE (UNII: TU7HW0W0QT) SODIUM PIDOLATE (UNII: 1V74VH163T) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) DISODIUM LAURETH SULFOSUCCINATE (UNII: D6DH1DTN7E) FRUCTOSE (UNII: 6YSS42VSEV) LINOLEIC ACID (UNII: 9KJL21T0QJ) OLEIC ACID (UNII: 2UMI9U37CP) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) TRIDECETH-9 (UNII: X9HD79I514) VETIVERIA ZIZANIOIDES ROOT (UNII: 37TB8LUP9Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62476-010-16 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/01/2023 Labeler - Industria La Popular, S.A. (846044576)