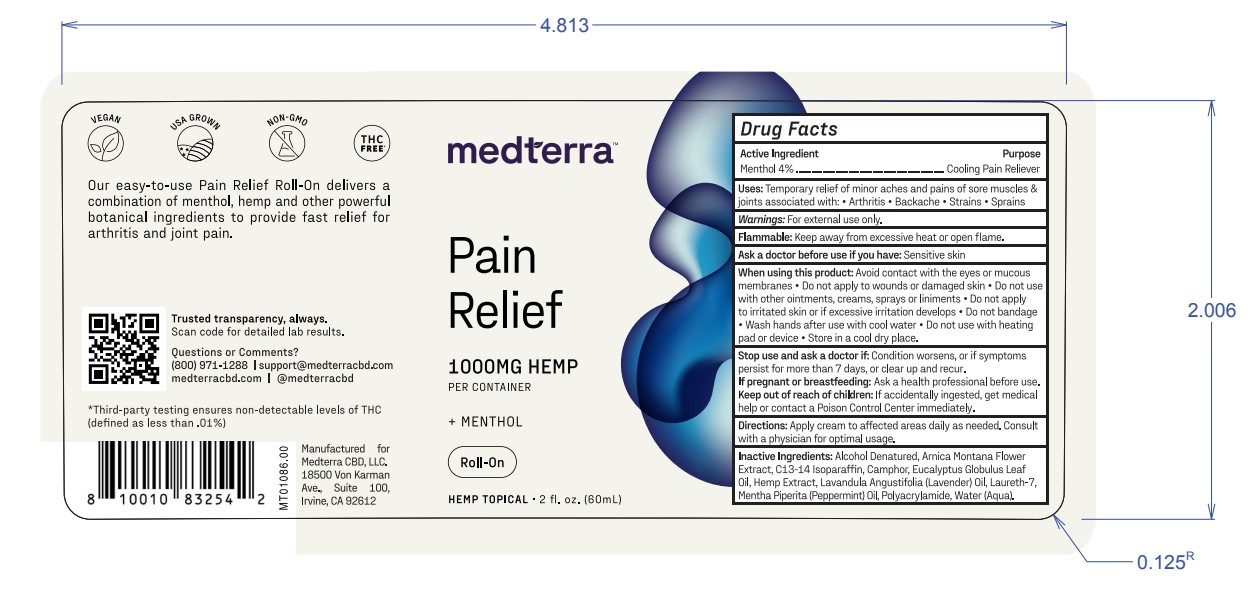
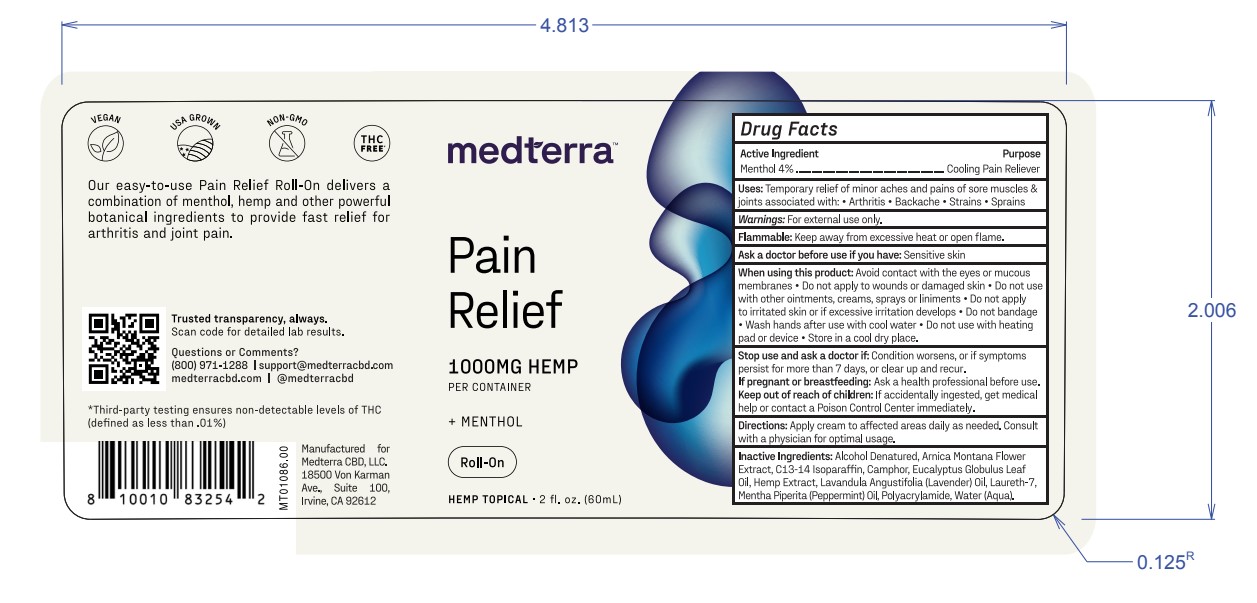
Label: MEDTERRA PAIN RELIEF MENTHOL ROLL-ON- menthol cream
- NDC Code(s): 83696-601-60
- Packager: Respect Manufacturing Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Warnings
- Purpose
- Uses
- Flammable
- Ask a doctor before use if you have
-
When using this product:
Avoid contact with the eyes or mucous
membranes • Do not apply to wounds or damaged skin • Do not use
with other ointments, creams, sprays orliniments •Do not apply
to irritated skin orif excessive irritation develops • Do not bandage
• Wash hands after use with cool water • Do not use with heating
pad or device • Store in a cool dry place. - Stop use and ask a doctor if:
- If pregnant or breastfeeding:
- Keep out of reach of children:
- Directions
- Inactive Ingredients
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MEDTERRA PAIN RELIEF MENTHOL ROLL-ON
menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83696-601 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 2.36 g in 59.1 g Inactive Ingredients Ingredient Name Strength EUCALYPTUS OIL (UNII: 2R04ONI662) WATER (UNII: 059QF0KO0R) PEPPERMINT OIL (UNII: AV092KU4JH) CAMPHOR OIL (UNII: 75IZZ8Y727) LAURETH-7 (UNII: Z95S6G8201) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) ALCOHOL (UNII: 3K9958V90M) POLYACRYLAMIDE (CROSSLINKED; 0.01-0.2 MOLE PERCENT BISACRYLAMIDE) (UNII: RHA9LWJ494) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) HEMP (UNII: TD1MUT01Q7) LAVENDER OIL (UNII: ZBP1YXW0H8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83696-601-60 59.1 g in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 01/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/01/2022 Labeler - Respect Manufacturing Corporation (118862975)