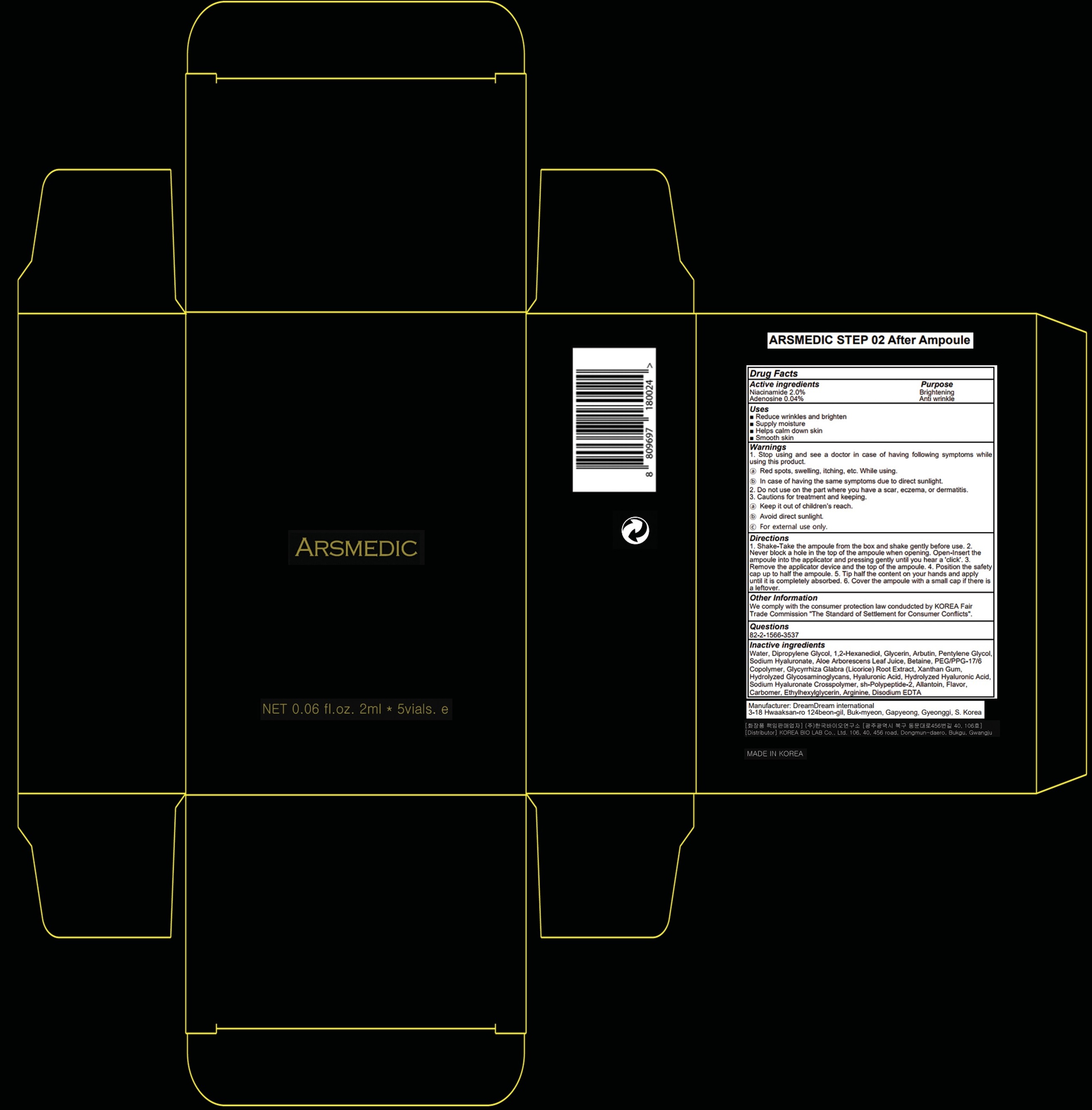
Label: ARSMEDIC STEP 02 AFTER AMPOULE- niacinamide, adenosine liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 82267-020-01, 82267-020-02 - Packager: Korea Bio Lab Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 24, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- PURPOSE
- Uses
-
WARNINGS
1. Stop using and see a doctor in case of having following symptoms while using this product.
ⓐ Red spots, swelling, itching, etc. While using.
ⓑ In case of having the same symptoms due to direct sunlight.
2. Do not use on the part where you have a scar, eczema, or dermatitis.
3. Cautions for treatment and keeping.
ⓐ Keep it out of children’s reach.
ⓑ Avoid direct sunlight.
ⓒ For external use only. - KEEP OUT OF REACH OF CHILDREN
-
Directions
1. Shake-Take the ampoule from the box and shake gently before use. 2. Never block a hole in the top of the ampoule when opening. Open-Insert the ampoule into the applicator and pressing gently until you hear a 'click'. 3. Remove the applicator device and the top of the ampoule. 4. Position the safety cap up to half the ampoule. 5. Tip half the content on your hands and apply until it is completely absorbed. 6. Cover the ampoule with a small cap if there is a leftover.
- Other Information
- QUESTIONS
-
INACTIVE INGREDIENTS
Water, Dipropylene Glycol, 1,2-Hexanediol, Glycerin, Arbutin, Pentylene Glycol, Sodium Hyaluronate, Aloe Arborescens Leaf Juice, Betaine, PEG/PPG-17/6 Copolymer, Glycyrrhiza Glabra (Licorice) Root Extract, Xanthan Gum, Hydrolyzed Glycosaminoglycans, Hyaluronic Acid, Hydrolyzed Hyaluronic Acid, Sodium Hyaluronate Crosspolymer, sh-Polypeptide-2, Allantoin, Flavor, Carbomer, Ethylhexylglycerin, Arginine, Disodium EDTA
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ARSMEDIC STEP 02 AFTER AMPOULE
niacinamide, adenosine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82267-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Niacinamide (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) Niacinamide 2.0 g in 100 mL Adenosine (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) Adenosine 0.04 g in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Dipropylene Glycol (UNII: E107L85C40) 1,2-Hexanediol (UNII: TR046Y3K1G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82267-020-02 5 in 1 CARTON 09/01/2021 1 NDC:82267-020-01 2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/01/2021 Labeler - Korea Bio Lab Co., Ltd (695889354) Registrant - Korea Bio Lab Co., Ltd (695889354) Establishment Name Address ID/FEI Business Operations DreamDream international 695625881 manufacture(82267-020)