Label: INFLUENZINUM (influenza a virus a/michigan/45/2015 x-275 (h1n1) hemagglutinin antigen (formaldehyde inactivated), influenza a virus a/hong kong/4801/2014 x-263b (h3n2) hemagglutinin antigen (formaldehyde inactivated), influenza b virus b/brisbane/60/2008 hemagglutinin antigen- formaldehyde inactivated pellet
-
Contains inactivated NDC Code(s)
NDC Code(s): 0220-2651-31, 0220-2651-41 - Packager: Laboratoires Boiron
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
Drug Label Information
Updated December 14, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- PURPOSE
- Use
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions, Comments?
- *
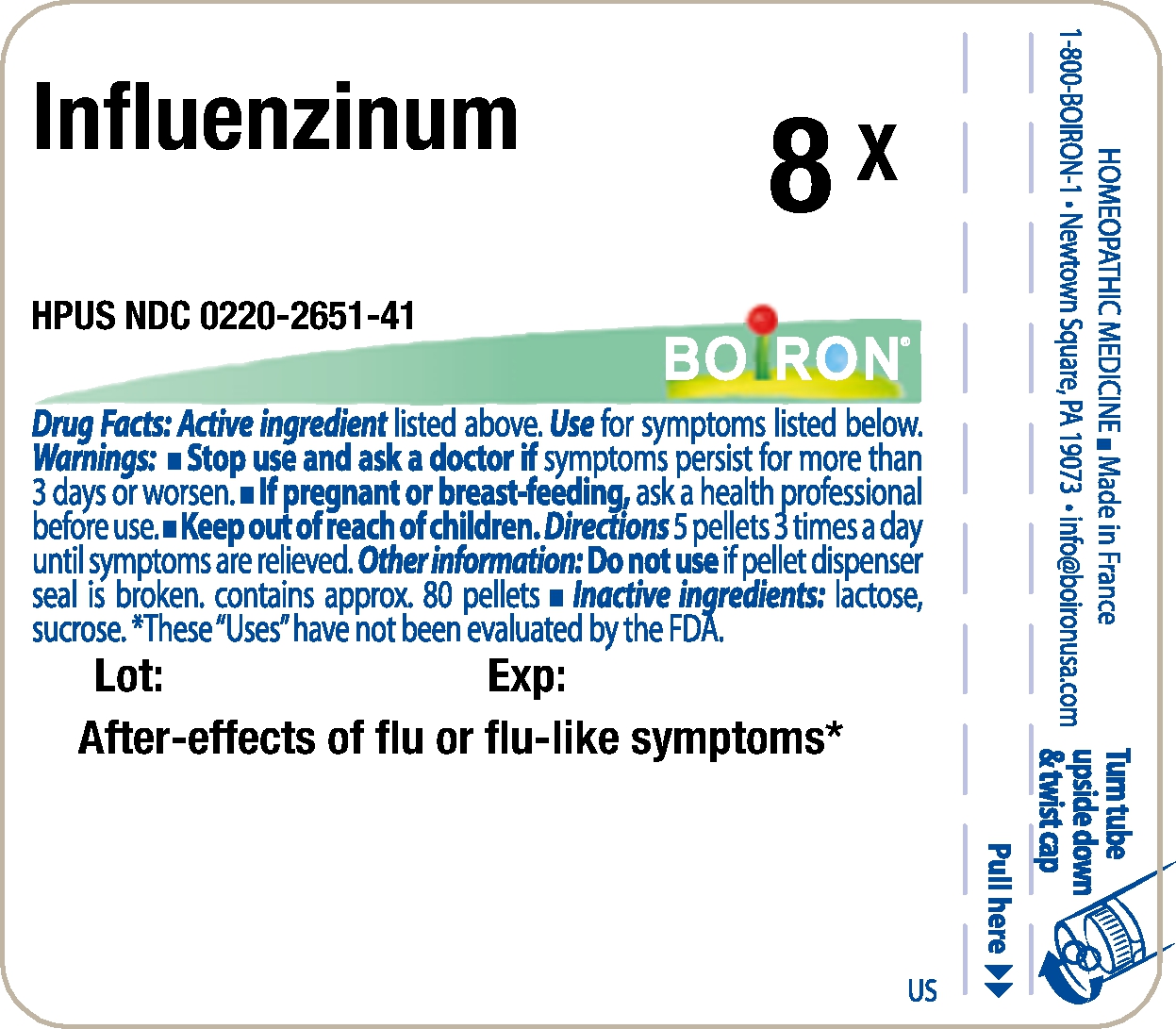
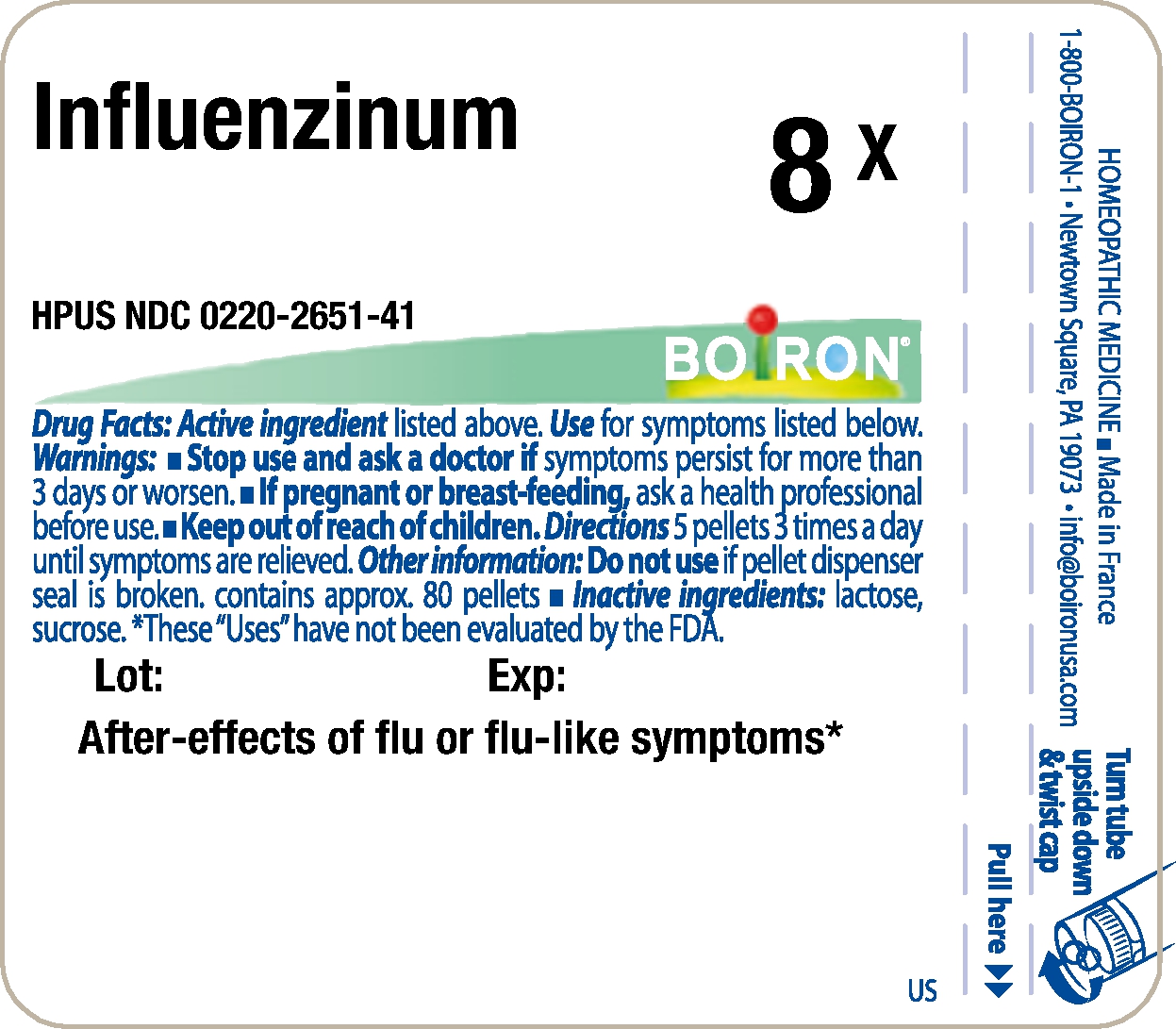
- Principle Display Panel - Influenzinum HPUS 8X
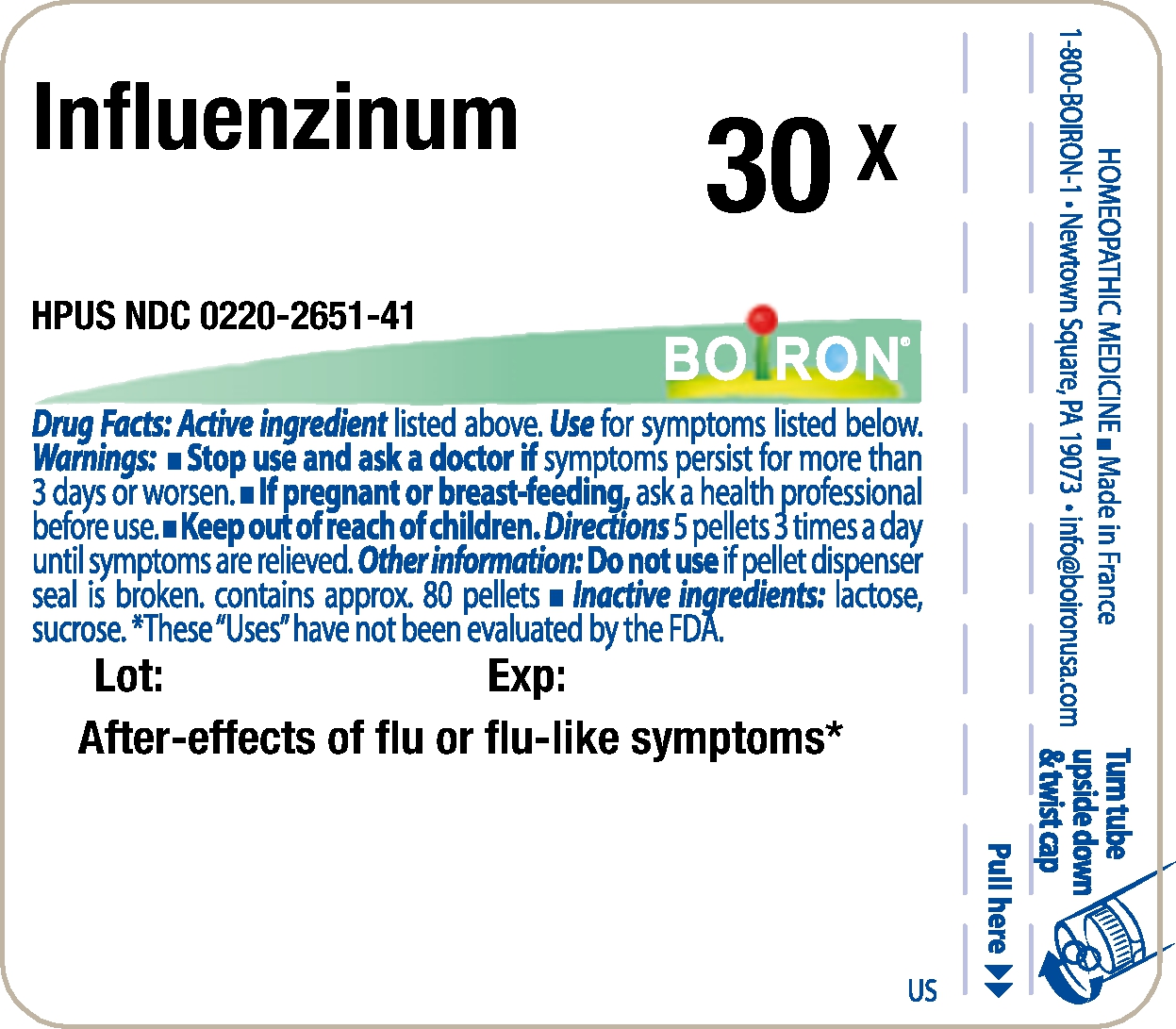
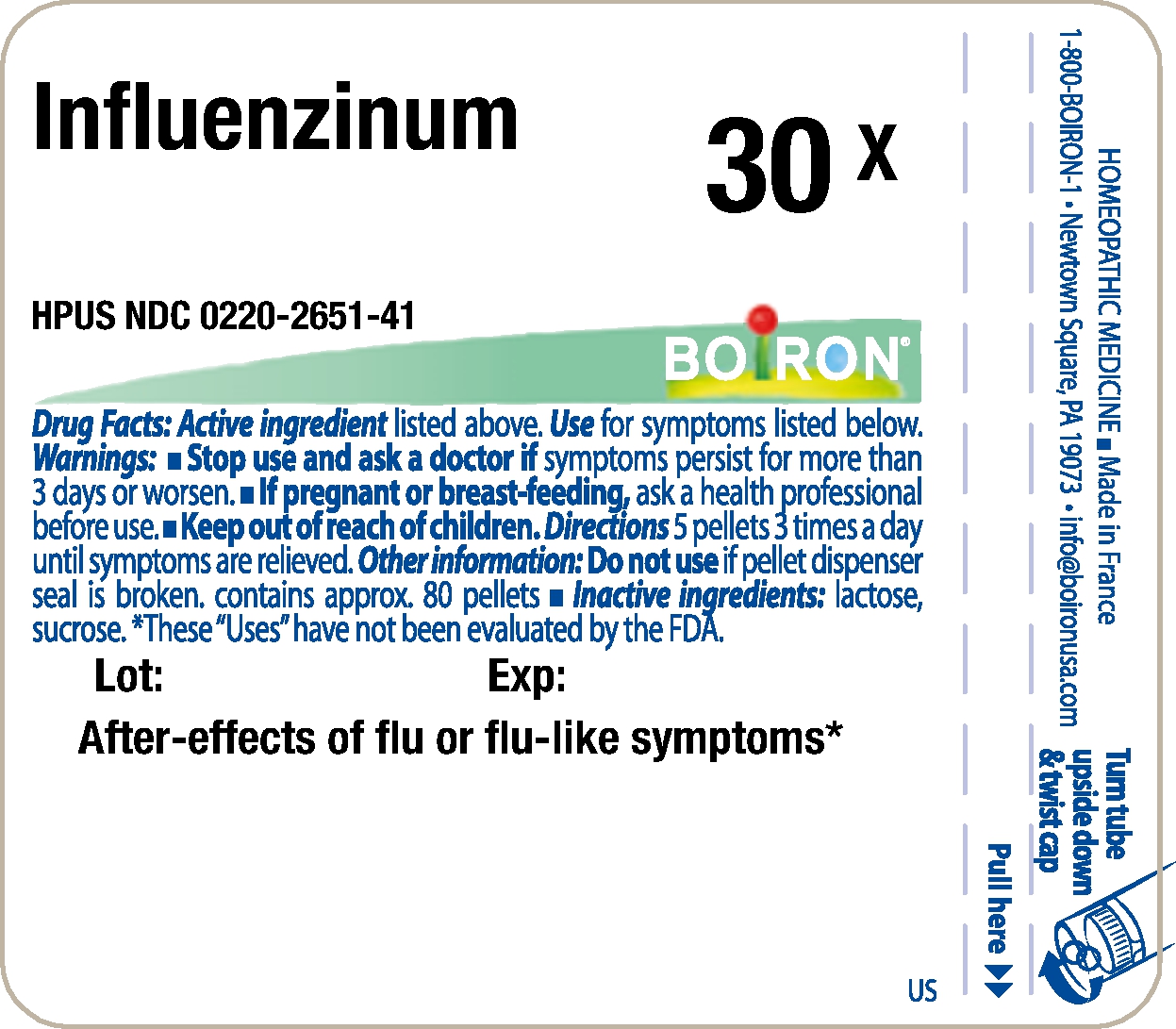
- Principle Display Panel - Influenzinum HPUS 30X
- Principle Display Panel - Influenzinum HPUS 6C
- Principle Display Panel - Influenzinum HPUS 9C
- Principle Display Panel - Influenzinum HPUS 12C
- Principle Display Panel - Influenzinum HPUS 15C
- Principle Display Panel - Influenzinum HPUS 30C
- Principle Display Panel - Influenzinum HPUS 200CK
- Principle Display Panel - Influenzinum HPUS 9C - Single Dose
-
INGREDIENTS AND APPEARANCE
INFLUENZINUM
influenza a virus a/michigan/45/2015 x-275 (h1n1) hemagglutinin antigen (formaldehyde inactivated), influenza a virus a/hong kong/4801/2014 x-263b (h3n2) hemagglutinin antigen (formaldehyde inactivated), influenza b virus b/brisbane/60/2008 hemagglutinin antigen (formaldehyde inactivated) pelletProduct Information Product Type VACCINE Item Code (Source) NDC:0220-2651 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INFLUENZA A VIRUS A/MICHIGAN/45/2015 X-275 (H1N1) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: 116749898H) (INFLUENZA A VIRUS A/MICHIGAN/45/2015 X-275 (H1N1) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:116749898H) INFLUENZA A VIRUS A/MICHIGAN/45/2015 X-275 (H1N1) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) 8 [hp_X] INFLUENZA A VIRUS A/HONG KONG/4801/2014 X-263B (H3N2) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: BAA3UN7VWD) (INFLUENZA A VIRUS A/HONG KONG/4801/2014 X-263B (H3N2) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:BAA3UN7VWD) INFLUENZA A VIRUS A/HONG KONG/4801/2014 X-263B (H3N2) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) 8 [hp_X] INFLUENZA B VIRUS B/BRISBANE/60/2008 HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: 166D8OWM7B) (INFLUENZA B VIRUS B/BRISBANE/60/2008 HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:166D8OWM7B) INFLUENZA B VIRUS B/BRISBANE/60/2008 HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) 8 [hp_X] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE (UNII: J2B2A4N98G) Product Characteristics Color white Score Shape ROUND Size 4mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0220-2651-41 80 in 1 TUBE; Type 0: Not a Combination Product 2 NDC:0220-2651-31 5 in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/03/1983 Labeler - Laboratoires Boiron (282560473) Registrant - Boiron Inc. (014892269) Establishment Name Address ID/FEI Business Operations Boiron 282560473 manufacture(0220-2651) Establishment Name Address ID/FEI Business Operations Boiron 380022322 manufacture(0220-2651)