Label: NICOTINAMIDE tablet
- NDC Code(s): 42192-374-60
- Packager: Acella Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
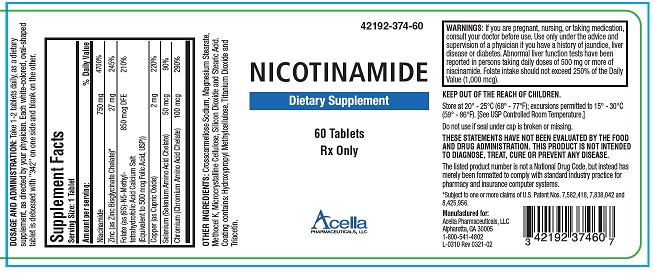
STATEMENT OF IDENTITY
RX Only
Dietary Supplement
DESCRIPTION: Nicotinamide Tablets is a prescription dietary supplement for oral administration, specifically formulated for the dietary management of patients with unique nutritional needs who require increased levels of one or more of the ingredients in this product. Each white-colored, oval-shaped tablet is debossed with “342” on one side and blank on the other.
Supplement Facts Serving Size: 1 Tablet Amount per serving: % Daily Value Nicotinamide 750 mg 4700% Zinc (as Zinc Bisglycinate Chelate)* 27 mg 245% Folate (as (6S)-N5-Methyl- tetrahydrofolic Acid Calcium Salt 850 mcg DFE 210% (Equivalent to 500 mcg Folic Acid, USP)) Copper (as Cupric Oxide) 2 mg 220% Selenium (Selenium Amino Acid Chelate) 50 mcg 90% Chromium (Chromium Amino Acid Chelate) 100 mcg 290% OTHER INGREDIENTS: Crosscarmellose Sodium, Magnesium Stearate, Methocel K, Microcrystalline Cellulose, Silicon Dioxide and Stearic Acid. Coating contains: Hydroxypropyl Methylcellulose, Titanium Dioxide and Triacetin.
INDICATIONS: Nicotinamide Tablets is indicated for use as a dietary supplement for patients who are deficient in, or who are at risk for deficiency in one or more of the ingredients in this product.
CONTRAINDICATIONS: This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
-
PRECAUTIONS
PRECAUTIONS: Large doses of Nicotinamide Tablets should be administered with caution in patients with a history of jaundice, liver disease, or diabetes. Patients with chronic liver failure and/or renal failure should exercise extreme caution in taking prescribed supplements containing copper.
Folic acid alone is improper treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid especially in doses above 1.0 mg daily may obscure pernicious anemia in that hematologic remission may occur while neurological manifestations remain progressive
-
WARNINGS
THESE STATEMENTS HAVE NOT BEEN EVALUATED BY THE FOOD AND DRUG ADMINISTRATION. THIS PRODUCT IS NOT INTENDED TO DIAGNOSE, TREAT, CURE, OR PREVENT ANY DISEASE.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
WARNINGS: If you are pregnant, nursing, or taking medication, consult your doctor before use. Use only under the advice and supervision of a physician if you have a history of jaundice, liver disease or diabetes. Abnormal liver function tests have been reported in persons taking daily doses of 500 mg or more of niacinamide. Folate intake should not exceed 250% of the Daily Value (1,000 mcg). PREGNANCY & NURSING MOTHERS:
Nicotinamide Tablets is not indicated for use as a prenatal/postnatal multivitamin for lactating and nonlactating mothers. Physicians and medical practitioners should administer Nicotinamide Tablets with caution to patients who are pregnant, lactating and/or taking medication.
ADVERSE REACTIONS: Allergic sensitization has been reported rarely following oral and parental administration of Folate.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION: Take 1-2 tablets daily, as a dietary supplement, as directed by your physician.
HOW SUPPLIED: Nicotinamide Tablets are white-colored, oval-shaped tablets debossed on one side with “342”, and are supplied in bottles of 60 tablets (42192-374-60). The listed product number is not a National Drug Code. Instead, Acella has assigned a product code formatted according to standard industry practice in order to comply with the formatting requirements of pharmacy and healthcare insurance computer systems.
STORAGE: Store at 20° - 25°C (68° - 77°F); excursions permitted to 15° - 30°C (59° - 86°F). [See USP Controlled Room Temperature.]
MANUFACTURED FOR:
Acella Pharmaceuticals, LLC Alpharetta, GA 30005
1-800-541-4802
L-0289 Rev 0321-01
*Subject to one or more claims of U.S. Patent Nos. 7,582,418, 7,838,042 and 8,425,956.
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NICOTINAMIDE
nicotinamide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42192-374 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 750 mg ZINC (UNII: J41CSQ7QDS) (ZINC - UNII:J41CSQ7QDS) ZINC 27 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 850 ug COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 2 mg SELENIUM (UNII: H6241UJ22B) (SELENIUM - UNII:H6241UJ22B) SELENIUM 50 ug CHROMIUM (UNII: 0R0008Q3JB) (CHROMIUM - UNII:0R0008Q3JB) CHROMIUM 100 ug Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE 2208 (100 MPA.S) (UNII: B1QE5P712K) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color WHITE Score no score Shape OVAL Size 23mm Flavor Imprint Code 342 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42192-374-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 05/25/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/25/2021 Labeler - Acella Pharmaceuticals, LLC (825380939) Registrant - Acella Pharmaceuticals, LLC (825380939)