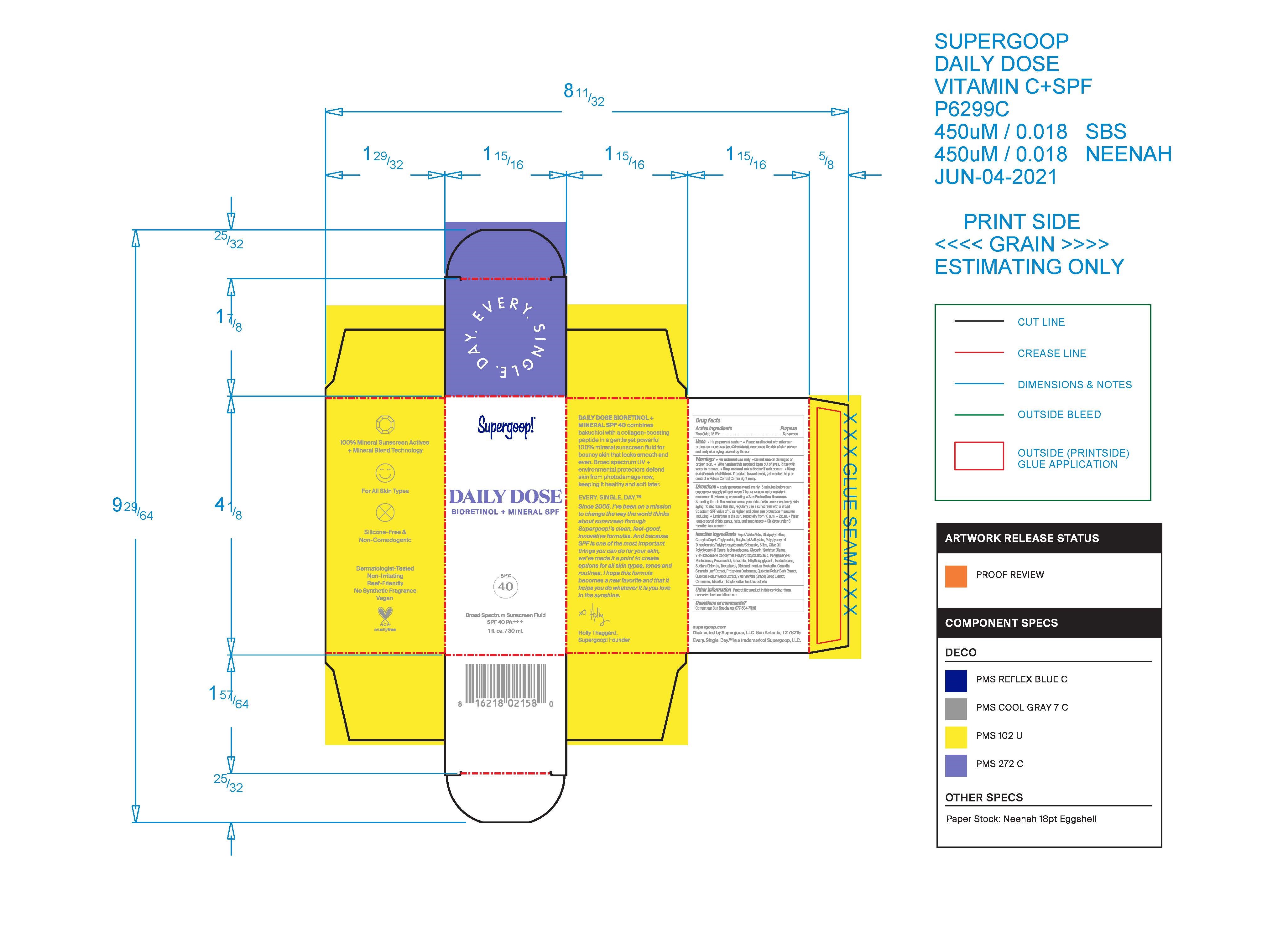
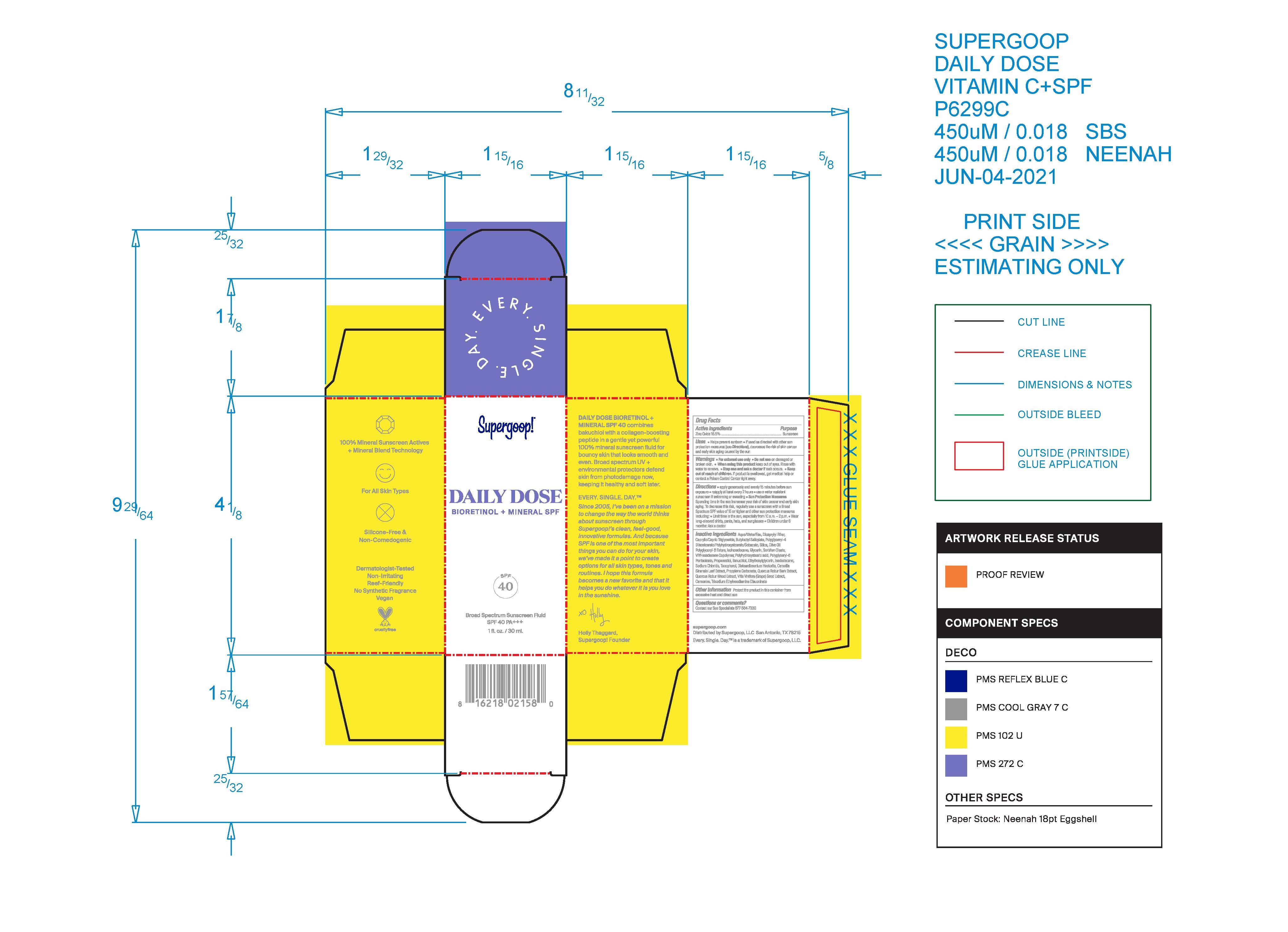
Label: DAILY SUPERGOOP DOSE BIORETINOL MINERAL SPF 40- daily dose bioretinol mineral spf 40 lotion
- NDC Code(s): 59735-614-01, 59735-614-02
- Packager: MANA Products, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- USES
- Warnings
- Keep out of the Reach of Children
-
Directions
• apply generously and evenly 15 minutes before sun exposure
• reapply at least every 2 hours
• use a water resistant sunscreen if swimming or sweating
Sun Protection Measures: Spending time in the sun increases your risk of skin
cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a
Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
◦ Limit time in the sun, especially from 10 a.m. - 2 p.m. ◦ Wear long-sleeved shirts,
pants, hats, and sunglasses • Children under 6 months: Ask a doctor -
Inactive Ingredients
Aqua/Water/Eau, Dicaprylyl Ether, Caprylic/Capric Triglyceride, Butyloctyl Salicylate,
Polyglyceryl-4 Diisostearate/Polyhydroxystearate/Sebacate, Silica, Olive Oil Polyglyceryl-6
Esters, Isohexadecane, Glycerin, Sorbitan Oleate, VP/Hexadecene Copolymer,
Polyhydroxystearic acid, Polyglyceryl-6 Pentaoleate, Propanediol, Bakuchiol,
Ethylhexylglycerin, Isododecane, Sodium Chloride, Tocopherol, Disteardimonium
Hectorite, Camellia Sinensis Leaf Extract, Propylene Carbonate, Quercus Robur Bark
Extract, Quercus Robur Wood Extract, Vitis Vinifera (Grape) Seed Extract, Carnosine,
Trisodium Ethylenediamine Disuccinate - Other Information
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DAILY SUPERGOOP DOSE BIORETINOL MINERAL SPF 40
daily dose bioretinol mineral spf 40 lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59735-614 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 16.5 g in 100 mL Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PEG-6 SORBITAN OLEATE (UNII: 58O7V09UCI) SODIUM CHLORIDE (UNII: 451W47IQ8X) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) VITIS VINIFERA SEED (UNII: C34U15ICXA) PROPANEDIOL (UNII: 5965N8W85T) BAKUCHIOL (UNII: OT12HJU3AR) QUERCUS ROBUR TWIG BARK (UNII: 2JFK226947) CAMELLIA SINENSIS FLOWER (UNII: 9I2BJY2J17) OLIVE OIL POLYGLYCERYL-6 ESTERS (UNII: 4KDO9AFM9I) VINYLPYRROLIDONE/HEXADECENE COPOLYMER (UNII: KFR5QEN0N9) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) POLYGLYCERYL-6 DIOLEATE (UNII: 062SZD3F3X) ISODODECANE (UNII: A8289P68Y2) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) QUERCUS ROBUR WOOD (UNII: 1B1CMC06QJ) ISOHEXADECANE (UNII: 918X1OUF1E) DICAPRYLYL ETHER (UNII: 77JZM5516Z) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) WATER (UNII: 059QF0KO0R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) GLYCERIN (UNII: PDC6A3C0OX) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) CARNOSINE (UNII: 8HO6PVN24W) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59735-614-01 1 in 1 BOX 10/18/2022 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:59735-614-02 6.5 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/18/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/18/2022 Labeler - MANA Products, INC (078870292) Establishment Name Address ID/FEI Business Operations MANA Products,Inc 032870270 manufacture(59735-614) Establishment Name Address ID/FEI Business Operations MANA Products, Inc 078870292 manufacture(59735-614)