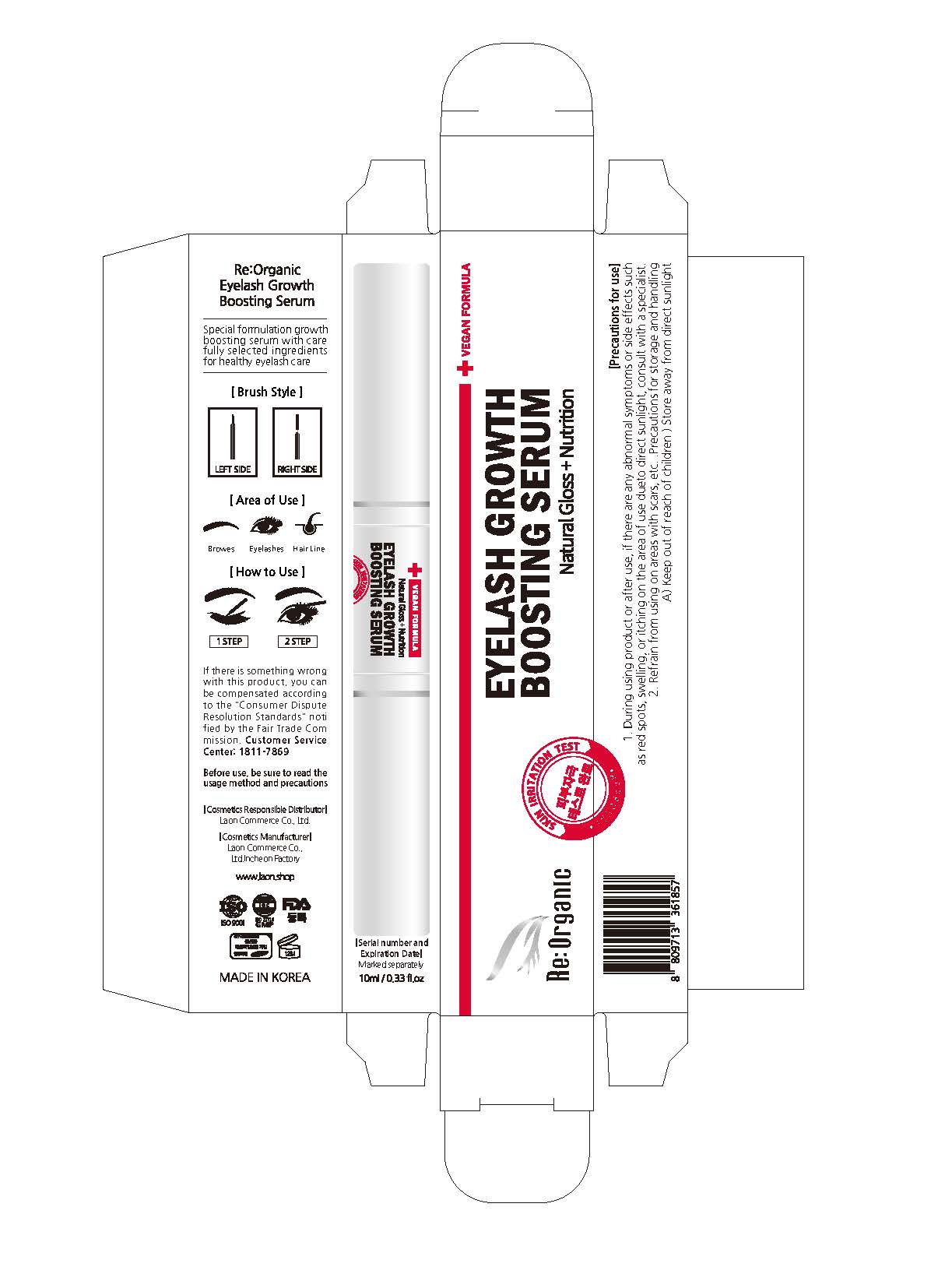
Label: REORGANIC EYELASH GROWTH BOOSTING SERUM- panthenol liquid
- NDC Code(s): 82083-0024-1
- Packager: LAON COMMERCE co ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Water
Butylene Glycol
Pentylene Glycol
1,2-Hexanediol
Sodium Hyaluronate
Glycerin
Ethylhexylglycerin
PEG-40 Hydrogenated Castor Oil
PPG-26-Buteth-26
Hydrogenated Lecithin
Caprylyl Glycol
Polyglyceryl-10 Stearate
Myristoyl Pentapeptide-17
Sodium Ascorbyl Phosphate
Tocopheryl Acetate
Glyceryl Linolenate
Glyceryl Arachidonate
Retinyl Palmitate
Biotin
Thiamine HCl
Folic Acid
Pyridoxine
Cyanocobalamin - PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
1. During using product or after use, if there are any abnormal symptoms or side effects such as red spots, swelling or itching in the area of use due to direct sunlight, consult with a specialist.
2. Refrain from using on areas with scars, etc.
3. Precautions for storage and handling
A) Keep out of reach of children
B) Store away from direct sunlight
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
REORGANIC EYELASH GROWTH BOOSTING SERUM
panthenol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82083-0024 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANTHENOL (UNII: WV9CM0O67Z) (PANTHENOL - UNII:WV9CM0O67Z) PANTHENOL 0.501 g in 100 mL Inactive Ingredients Ingredient Name Strength BIOTIN (UNII: 6SO6U10H04) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82083-0024-1 10 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 09/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/01/2023 Labeler - LAON COMMERCE co ltd (557839830) Registrant - LAON COMMERCE co ltd (557839830) Establishment Name Address ID/FEI Business Operations LAON COMMERCE CO Ltd 557839830 manufacture(82083-0024) , label(82083-0024)