Label: CPDA-1- anticoagulant citrate phosphate dextrose adenine solution
- NDC Code(s): 0942-6326-04
- Packager: Fenwal, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 27, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
4R3332E, 4R3327EFresenius KabiFenwal Blood-Pack Units Rx onlyUsing Anticoagulant Citrate Phosphate Dextrose Adenine Solution, USP (CPDA-1) with an Integral Sepacell RS-2000 Whole Blood Leukocyte Reduction Filter and Fenwal HighFlo Needle
Contains Fenwal Express System and Sample Diversion System for the collection of whole blood samples for laboratory testing. Also contains empty Transfer Pack containers for blood component aliquots.
Integral filter unit intended for leukocyte reduction of Whole Blood up to 8 hours after blood collection when Whole Blood is stored at ambient temperatures or up to 72 hours after blood collection when Whole Blood is refrigerated. The leukocyte reduced blood products may then be stored for the maximum allowable dating period.
Instructions for Use
Collection Procedure:
Use aseptic technique.
Notes:
- • If Sample Diversion System is not used, donor samples may be collected using an alternate method following standard procedures.
- • Nominal tubing dimensions of product are 0.118" inner diameter x 0.025" wall thickness.
Precautions:
- • Upon removal of Blood-Pack unit from the clear plastic overwrap, visually inspect the unit.
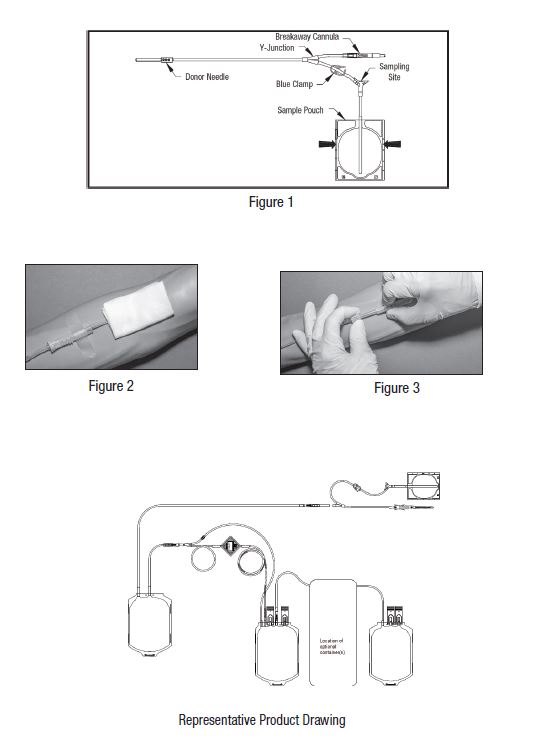
- • Do not use the product if the in-line cannula is broken and/or anticoagulant is present in the sample pouch or in the tubing from the in-line cannula to the sample pouch and donor needle (see Figure 1). Note that condensation in the empty tubing of the Blood-Pack unit is expected as a result of the sterilization process.
- • Do not use unless the solutions are clear.
1. Identify Blood-Pack unit using appropriate donor identification system.
2. Donor scale
- • Adjust donor scale to desired collection weight.
- • Position primary container on the donor scale as far as possible below donor arm.
3. Clamp donor tubing between the HighFlo1 needle and Y-junction with a clamp. (This step can be performed prior to step 1 or 2.)
4. Visually inspect the tubing from the in-line cannula to the sample pouch and donor needle, as well as the sample pouch to reconfirm that there is no anticoagulant present.
Note: Ensure that the sample pouch remains below the donor’s arm.
5. Following blood center procedures, apply pressure to donor’s arm and disinfect site of venipuncture.
6. Remove needle cover per instructions below:
- • Holding the hub and cover near the tamper-evident seal, twist cover and hub in opposite directions to break seal.
- • Remove needle cover, being careful not to drag the cover across the needle point.
7. Following blood center procedures, perform venipuncture, appropriately secure donor needle and/or tubing and release clamp.
8. When good blood flow is established, stabilize the front of the needle guard to arm with tape. (see Figure 2)
9. Allow the sample pouch to fill with blood according to center procedure. Monitor blood flow into sample pouch.
Notes:
- • The sample pouch contains an average fill volume of approximately 53 mL with a maximum fill volume of approximately 60 mL when filled to capacity.
- • If less blood sample volume is required, the flow to the sample pouch may be stopped prior to completely filling the pouch. For example, in order to target a fill volume of approximately 40 mL, fill to the level indicated by the arrows in Figure 1. Ensure the pouch is hanging vertically.
- • The tube leading from the Y-junction to the sample pouch contains an additional volume of approximately 2 mL.
Precautions:
- • Do not elevate or squeeze the sample pouch as this could cause blood to backflow from the sample pouch into the collection system.
- • Once the sample pouch is filled to desired volume, complete steps 10 - 18 within approximately 4 minutes to avoid possible clot formation in the tubing and/or sample pouch.
10. Close the blue clamp on tubing between the Y-junction and the sample pouch.
11. Break the in-line cannula below the Y-junction in the donor tubing to the primary container allowing blood collection to proceed. To completely break the in-line cannula, grasp with both hands. Snap it at a 90° angle in one direction, and then bend it at a 90° angle in the
opposite direction. Ensure the in-line cannula is completely broken and that the blood flows freely to the primary container.
Precaution: Failure to break the in-line cannula completely may result in restricted blood flow.
12. Mix blood and anticoagulant in the primary container immediately, at several intervals during collection, and immediately after collection.
13. Following blood center procedures, hermetically seal the tubing between the sampling site and the Y-junction to maintain sterility of the blood collection system prior to removing blood samples.
Warning:
- • Do not proceed with the remaining steps until the tubing leading to the sample pouch is hermetically sealed between the sampling site and the Y-junction. To maintain the whole blood collection container as a closed system, the tubing between the sample pouch and Y-junction must be hermetically sealed prior to inserting the access device into the sampling site. Failure to do so may lead to contamination of the whole blood collection.
14. To collect samples, insert the access device by pushing firmly into the sampling site until the membrane seal is penetrated.
Note: If the access device is assembled such that the outer barrel is screwed onto the Luer, make sure to rotate clockwise upon insertion to avoid barrel detaching from Luer.
15. Open the cap on the access device (if applicable). Hold access device so that the sample pouch hangs down.
16. Directly align the vacuum sample tube with the internal needle in the access device. Insert vacuum sample tube into device.
17. Allow vacuum sample tube to fill with blood then remove from the access device.
18. Repeat steps 16 and 17 until the desired number of vacuum sample tubes have been filled.
Notes:
- • If the access device needs to be replaced, clamp the tubing between the sampling site and the sample pouch. Then, grasp base of sampling site with one hand and pull the access device out with the other hand. Firmly insert the new access device. Remove clamp and continue sampling.
- • If the access device is assembled such that the outer barrel is screwed onto the Luer, make sure to rotate clockwise upon removal to avoid barrel detaching from Luer.
- • The access device can only be replaced one time.
Precaution: When replacing access device, be careful to avoid contact with any blood droplets on the Luer or sampling site. Discard used access device appropriately.
19. Collect the appropriate volume based on Blood-Pack unit used. Note: The volume of anticoagulant is sufficient for the blood collection indicated on Blood-Pack unit ± 10%.
Precaution: Once the desired blood volume is collected, complete steps 20-24 within approximately 4 minutes to avoid possible clot formation in the tubing.
20. Release pressure on the donor’s arm. If appropriate, apply clamp to donor tubing between the needle and the Y-junction.
21. Hermetically seal donor tubing between the in-line cannula and the primary container.
22. Withdrawal of Needle (see Figure 3)
Precaution: The needle guard must be held stationary while the needle is withdrawn into it.
a) Place folded sterile gauze over puncture site and hold in place with finger tip without exerting pressure.
b) Hold sides of needle guard near the front, between the index finger and thumb. Pull the hub back smoothly until the needle is completely enclosed and securely locked into the needle guard.
c) Confirm the needle is completely enclosed and securely locked into the needle guard.
23. Remove and discard the Sample Diversion System and needle in needle guard into an appropriate biohazardous waste container following established procedures. If the donor tubing is also to be discarded, hermetically seal donor tubing directly above the primary
container and remove.
Note: Step 24 may be performed prior to step 23 if desired.
24. If the donor tubing is not sealed directly above the primary container, then strip the blood from the remaining donor tubing into the primary container. Mix and allow the tubing to refill; repeat once.
Filtration Procedure:
Precaution: Whole blood collected from certain donors may have extended filtration times and the potential for ineffective filtration and leukoreduction.
Note: The time of Whole Blood filtration may vary depending on processing option selected.
- a) Within 8 hours of collection if Whole Blood is held at ambient temperature.
- b) Within 72 hours of collection if Whole Blood is refrigerated following collection.
25. Mix unfiltered Whole Blood thoroughly. Invert the primary container and hang thefilter set such that the filter remains vertical. To achieve maximum flow rate, allow set to hang to full length.
Note: The filtered Whole Blood container must remain below the level of the filter during filtration. For proper air expression to occur, ensure the filtered Whole Blood container is vertical.
26. Inspect all tubing to insure it hangs freely without kinks. Install and close clamp on bypass line.
27. Break the in-line cannula above the filter to start filtration. To completely break the in-line cannula, grasp with both hands. Snap it at a 90° angle in one direction, and then bend it at a 90° angle in the opposite direction. Allow filtration to continue until flow stops.
Note: Manual or mechanical pressure should not be used to increase the flow rate through the filter.
Note: Tubing below the filter should not be stripped at any time during the filtration process.
Note: If the filtration of Whole Blood is initiated at ambient temperature and not completed within 8 hours after blood collection, then filtration should be completed between 1 and 6°C.
Note: If recovery of residual blood in the primary container is desired, install and close a clamp on the segment line prior to removing the clamp on the bypass line. The segment line clamp can then be removed after step 28.
28. Open the clamp on the bypass line and allow air to transfer from the filtered Whole Blood container to the primary container.
Note: If desired, gently squeeze the filtered Whole Blood container to transfer remaining air through the bypass line.
29. Allow filtration to continue until the inlet side of the filter is filled with air.
30. Hermetically seal and separate the bypass line above the filtered Whole Blood container. Also hermetically seal and separate the segment line tubing directly above the top donor segment number. Use care to avoid fluid splatter. Discard filter and primary container
appropriately.
31. Make donor segments. Leave segments attached to the filtered Whole Blood container.
Component Preparation Procedure:
Note: Platelet concentrates are not intended to be made with this product.
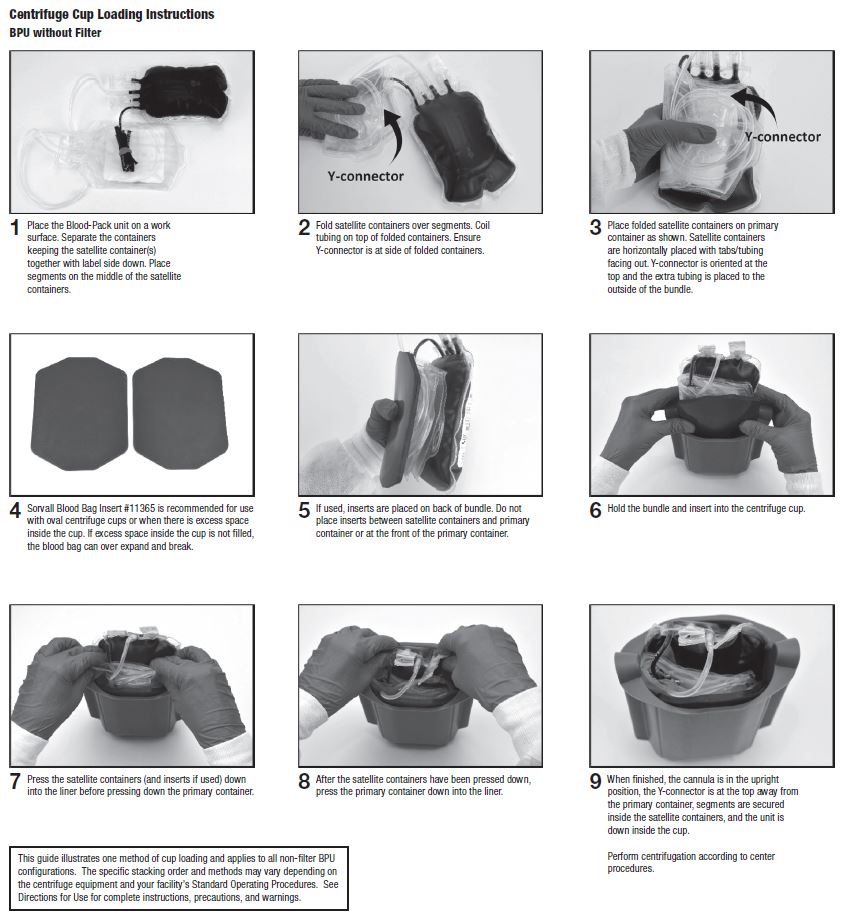
32. At the appropriate time, prepare the Blood-Pack unit for centrifugation by thoroughly mixing the primary container end over end, then load the unit in a centrifuge cup per the instructions on page 3.
33. Following centrifugation, remove containers from the centrifugation cup taking care not to disturb the red blood cell / plasma interface.
34. Place filtered Whole Blood container in plasma extractor. Clamp tubing to Transfer Pack containers that are not to be filled with plasma. Express plasma into empty Transfer Pack container(s) by releasing pressure plate and breaking cannula at the top of filtered Whole Blood container.
35. When the desired amount of plasma has been removed, clamp the tubing leading to the plasma container(s). Hermetically seal and separate transfer tubing(s) being careful to avoid fluid splatter. Detach filled containers as desired.
Note: If component aliquots are to be made at a later time, do not seal tubing between the filled container(s) and empty transfer packs. Leave tubing between containers clamped to prevent inadvertent fluid transfer. If desired, remove clamp(s) and transfer portions of the blood components into empty Transfer Pack container(s). Hermetically seal and separate transfer tubing(s) being careful to avoid fluid splatter. Detach filled containers as desired.
36. Label each blood component following blood center procedures.
37. For further processing of blood components with multiple Blood-Pack units, use standard component processing and storage techniques.
Note: Fresh Frozen Plasma should be separated from the Red Blood Cells and placed in the freezer at -18°C or colder within 8 hours after blood collection.
38. Store suspended CPDA-1 Whole Blood/Red Blood Cells, Leukocytes Reduced between 1 and 6°C.
39. Infuse CPDA-1 Whole Blood/Red Blood Cells, Leukocytes Reduced within 35 days of collection.
Warning: Failure to achieve closed system processing conditions negates the extended storage claim and the red blood cell product must be transfused within 24 hours.
Store at Controlled Room Temperature. Protect from freezing. Avoid excessive heat.
Definition of “Controlled Room Temperature”:
“A temperature maintained thermostatically that encompasses the usual and customary working environment of 20° to 25°C (68° to 77°F); that results in a mean kinetic temperature calculated to be not more than 25°C; and that allows for excursions between 15°C and 30°C (59° and 86°F) that are experienced in pharmacies, hospitals, and warehouses. Provided the mean kinetic temperature remains in the allowed range, transient spikes up to 40°C are permitted as long as they do not exceed 24 hours ...
The mean kinetic temperature is a calculated value that may be used as an isothermal storage temperature that simulates the non isothermal effects of storage temperature variations.”
Reference: United States Pharmacopeia, General Notices. United States
Pharmacopeial Convention, Inc. 12601 Twinbrook Parkway, Rockville, MD.
1 Van der Meer, P.F., & de Korte, D. “Increase of blood donation speed by optimizing the needle-to-tubing connection: an application of donation software.” Vox Sanguinis 2009, 97: 21-25
Sorvall is a trademark of Thermo Fisher Scientific LLC.
Sepacell is a trademark of Asahi Kasei Medical Co., Ltd.
© 2019 Fresenius Kabi AG. All rights reserved
47-23-13-836 REV: A
-
PACKAGE/LABEL DISPLAY PANEL
Code 4R3332E
10 Units
Fresenius Kabi
Fenwal Blood-Pack Units Quadruple
Anticoagulant Citrate Phosphate Dextrose Adenine Solution, USP (CPDA-1) Blood-Pack Unit; Integral Sepacell RS-2000 Whole Blood Leukocyte Reduction Filter
For Collection and Processing of 500 mL Blood
Fenwal Express System, Sample Diversion System, 16 ga. Ultra Thin Wall HighFLo Needle, and three empty 400 mL Transfer-Pack containers for blood component aliquots.
Rx only
Each unit consists of a PL 146 Plastic primary container with 70 mL of CPDA-1 solution containing 2.23 g Dextrose (monohydrate) USP, 1.84 g Sodium Citrate (dihydrate) USP, 209 mg Citric Acid (anhydrous) USP, 155 mg Monobasic Sodium Phosphate (monohydrate) USP and 19.3 mg Adenine USP, pH may have been adjusted with sodium hydroxide; an integral RS-2000 filter with one empty 450 mL container for red cell storage and three empty 400 mL Transfer-Pack containers.
Sterile, non-pyrogenic fluid path
See instructions for use.Single use only.
Store at Controlled Room Temperature (refer to direction insert). Protect from freezing. Avoid excessive heat.
- •
- Open pouch by tearing across at notch.
- •
- Direct handling of product surfaces prior to extended storage in the foil pouch, may result in mold growth.
- •
- Unused units in open foil pouch may be kept up to 60 days by folding and securing open end of foil pouch to prevent possible loss of moisture, provided:
- I)
- Units are not removed from foil pouch, or
- II)
- Unused units removed from foil pouch are returned to the foil pouch within 12 hours. Units may be removed from the pouch and returned only once.
- •
- Units removed from the foil pouch (that are not returned to the pouch within 12 hours) must be used within 4 days (96 hours). Units out of the foil pouch for longer than 96 hours must be discarded.
Sepacell is a trademark of Asahi Kasei Medical Co., Ltd.
Manufacturer
Fresenius Kabi AG
61346 Bad Homburg / Germany
www.fresenius-kabi.com
Made in US
47-28-13-844 REV: A
-
INGREDIENTS AND APPEARANCE
CPDA-1
anticoagulant citrate phosphate dextrose adenine solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0942-6326 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dextrose Monohydrate (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) Dextrose Monohydrate 2.23 g in 70 mL Trisodium Citrate Dihydrate (UNII: B22547B95K) (Anhydrous Citric Acid - UNII:XF417D3PSL) Anhydrous Citric Acid 1.84 g in 70 mL Anhydrous Citric Acid (UNII: XF417D3PSL) (Anhydrous Citric Acid - UNII:XF417D3PSL) Anhydrous Citric Acid 209 mg in 70 mL Sodium Phosphate, Monobasic, Monohydrate (UNII: 593YOG76RN) (PHOSPHATE ION - UNII:NK08V8K8HR) Sodium Phosphate, Monobasic, Monohydrate 155 mg in 70 mL Adenine (UNII: JAC85A2161) (Adenine - UNII:JAC85A2161) Adenine 19.3 mg in 70 mL Inactive Ingredients Ingredient Name Strength Sodium Hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0942-6326-04 70 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN770420 12/15/2008 Labeler - Fenwal, Inc. (794519020) Establishment Name Address ID/FEI Business Operations Fenwal International, Inc. 091164590 MANUFACTURE(0942-6326)