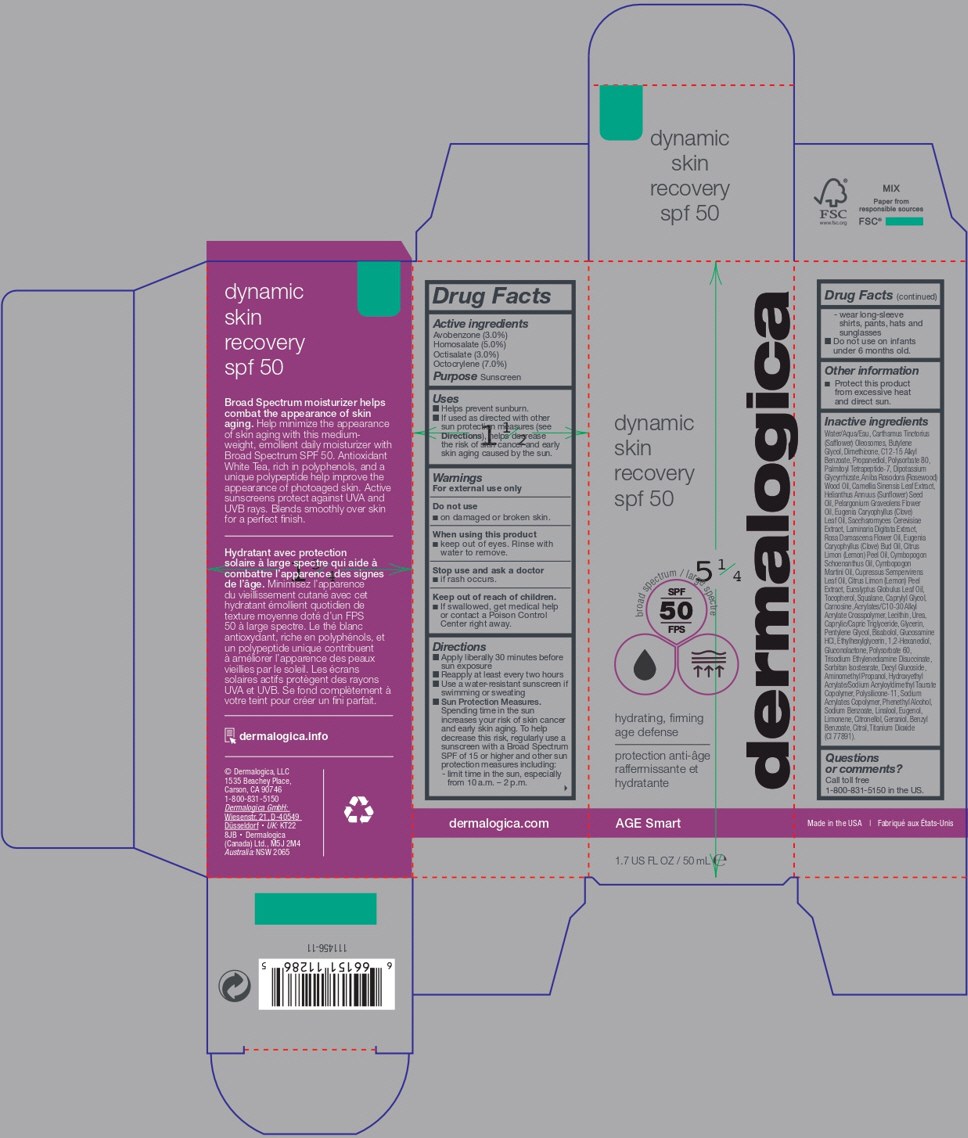
Label: DERMALOGICA DYNAMIC SKIN RECOVERY SPF 50- sunscreen lotion
-
NDC Code(s):
68479-382-00,
68479-382-01,
68479-382-02,
68479-382-03, view more68479-382-04, 68479-382-41
- Packager: Dermalogica, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
-
Directions
Apply liberally 30 minutes before
sun exposure
Reapply at least every two hours
Use a water-resistant sunscreen if
swimming or sweating
Sun Protection Measures.
Spending time in the sun
increases your risk of skin cancer
and early skin aging. To help
decrease this risk, regularly use a
sunscreen with a Broad Spectrum
SPF of 15 or higher and other sun
protection measures including:
- limit time in the sun, especially
from 10 a.m. – 2 p.m.- wear long-sleeve
shirts, pants, hats and
sunglasses
Do not use on infants
under 6 months old. - Other information
-
Inactive ingredients
Water/Aqua/Eau, Carthamus Tinctorius
(Safflower) Oleosomes, Butylene
Glycol, Dimethicone, C12-15 Alkyl
Benzoate, Propanediol, Polysorbate 80,
Palmitoyl Tetrapeptide-7, Dipotassium
Glycyrrhizate, Aniba Rosodora (Rosewood)
Wood Oil, Camellia Sinensis Leaf Extract,
Helianthus Annuus (Sunflower) Seed
Oil, Pelargonium Graveolens Flower
Oil, Eugenia Caryophyllus (Clove)
Leaf Oil, Saccharomyces Cerevisiae
Extract, Laminaria Digitata Extract,
Rosa Damascena Flower Oil, Eugenia
Caryophyllus (Clove) Bud Oil, Citrus
Limon (Lemon) Peel Oil, Cymbopogon
Schoenanthus Oil, Cymbopogon
Martini Oil, Cupressus Sempervirens
Leaf Oil, Citrus Limon (Lemon) Peel
Extract, Eucalyptus Globulus Leaf Oil,
Tocopherol, Squalane, Caprylyl Glycol,
Carnosine, Acrylates/C10-30 Alkyl
Acrylate Crosspolymer, Lecithin, Urea,
Caprylic/Capric Triglyceride, Glycerin,
Pentylene Glycol, Bisabolol, Glucosamine
HCl, Ethylhexylglycerin, 1,2-Hexanediol,
Gluconolactone, Polysorbate 60,
Trisodium Ethylenediamine Disuccinate,
Sorbitan Isostearate, Decyl Glucoside,
Aminomethyl Propanol, Hydroxyethyl
Acrylate/Sodium Acryloyldimethyl Taurate
Copolymer, Polysilicone-11, Sodium
Acrylates Copolymer, Phenethyl Alcohol,
Sodium Benzoate, Linalool, Eugenol,
Limonene, Citronellol, Geraniol, Benzyl
Benzoate, Citral, Titanium Dioxide
(CI 77891). - Questionsor comments?
- dermalogica dynamic skin recovery spf 50 - outer label
- dermalogica dynamic skin recovery spf 50 - inner label
-
INGREDIENTS AND APPEARANCE
DERMALOGICA DYNAMIC SKIN RECOVERY SPF 50
sunscreen lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68479-382 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 5 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 3 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL Inactive Ingredients Ingredient Name Strength PELARGONIUM GRAVEOLENS FLOWER OIL (UNII: 3K0J1S7QGC) CLOVE LEAF OIL (UNII: VCA5491KVF) LAMINARIA DIGITATA (UNII: 15E7C67EE8) SACCHAROMYCES CEREVISIAE (UNII: 978D8U419H) ROSA X DAMASCENA FLOWER OIL (UNII: 18920M3T13) CLOVE OIL (UNII: 578389D6D0) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) CUPRESSUS SEMPERVIRENS LEAF OIL (UNII: M7QUY89S4O) PALMAROSA OIL (UNII: 0J3G3O53ST) CYMBOPOGON SCHOENANTHUS OIL (UNII: XE7K568ILO) LEMON PEEL (UNII: 72O054U628) EUCALYPTUS OIL (UNII: 2R04ONI662) SUNFLOWER OIL (UNII: 3W1JG795YI) TOCOPHEROL (UNII: R0ZB2556P8) SQUALANE (UNII: GW89575KF9) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) CARNOSINE (UNII: 8HO6PVN24W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) UREA (UNII: 8W8T17847W) CAPRYLIC/CAPRIC/LAURIC TRIGLYCERIDE (UNII: FJ1H6M2JG9) GLYCERIN (UNII: PDC6A3C0OX) PENTYLENE GLYCOL (UNII: 50C1307PZG) .ALPHA.-BISABOLOL, (+)- (UNII: 105S6I733Z) GLUCOSAMINE HYDROCHLORIDE (UNII: 750W5330FY) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GLUCONOLACTONE (UNII: WQ29KQ9POT) POLYSORBATE 60 (UNII: CAL22UVI4M) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) SODIUM ACRYLATE/STYRENE SULFONATE COPOLYMER (11000 MW) (UNII: N30934870L) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) SODIUM BENZOATE (UNII: OJ245FE5EU) LINALOOL, (+)- (UNII: F4VNO44C09) EUGENOL (UNII: 3T8H1794QW) LIMONENE, (+)- (UNII: GFD7C86Q1W) .BETA.-CITRONELLOL, (+/-)- (UNII: 565OK72VNF) GERANIOL (UNII: L837108USY) BENZYL BENZOATE (UNII: N863NB338G) CITRAL (UNII: T7EU0O9VPP) WATER (UNII: 059QF0KO0R) CARTHAMUS TINCTORIUS SEED OLEOSOMES (UNII: 9S60Q72309) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE (UNII: 92RU3N3Y1O) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PROPANEDIOL (UNII: 5965N8W85T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) ROSEWOOD OIL (UNII: F2522O5L7B) GREEN TEA LEAF (UNII: W2ZU1RY8B0) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68479-382-02 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/17/2022 2 NDC:68479-382-01 12 mL in 1 TUBE; Type 0: Not a Combination Product 01/17/2022 3 NDC:68479-382-04 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/17/2022 10/01/2023 4 NDC:68479-382-41 118 mL in 1 TUBE; Type 0: Not a Combination Product 10/01/2023 5 NDC:68479-382-03 1 in 1 CARTON 10/01/2023 5 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 6 NDC:68479-382-00 2 mL in 1 POUCH; Type 0: Not a Combination Product 10/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 01/17/2022 Labeler - Dermalogica, LLC (177698560) Establishment Name Address ID/FEI Business Operations Cosway 052400223 manufacture(68479-382) Establishment Name Address ID/FEI Business Operations McKenna Labs, Inc. 090631412 manufacture(68479-382)