Label: NMC MAGIC SERUM- adenosine liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 59220-3001-1 - Packager: NMC (Natural Magma Cosmetics)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated July 11, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
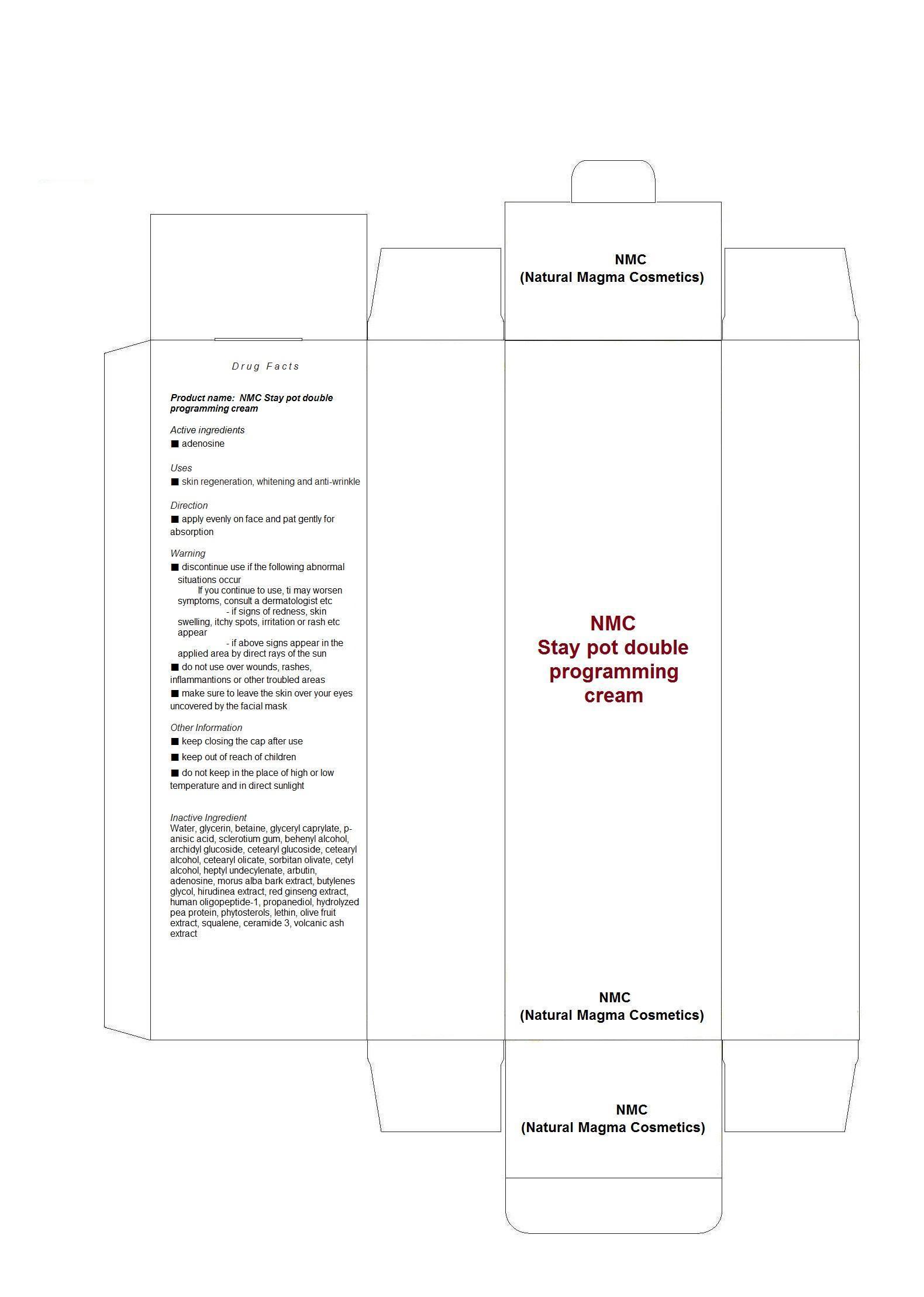
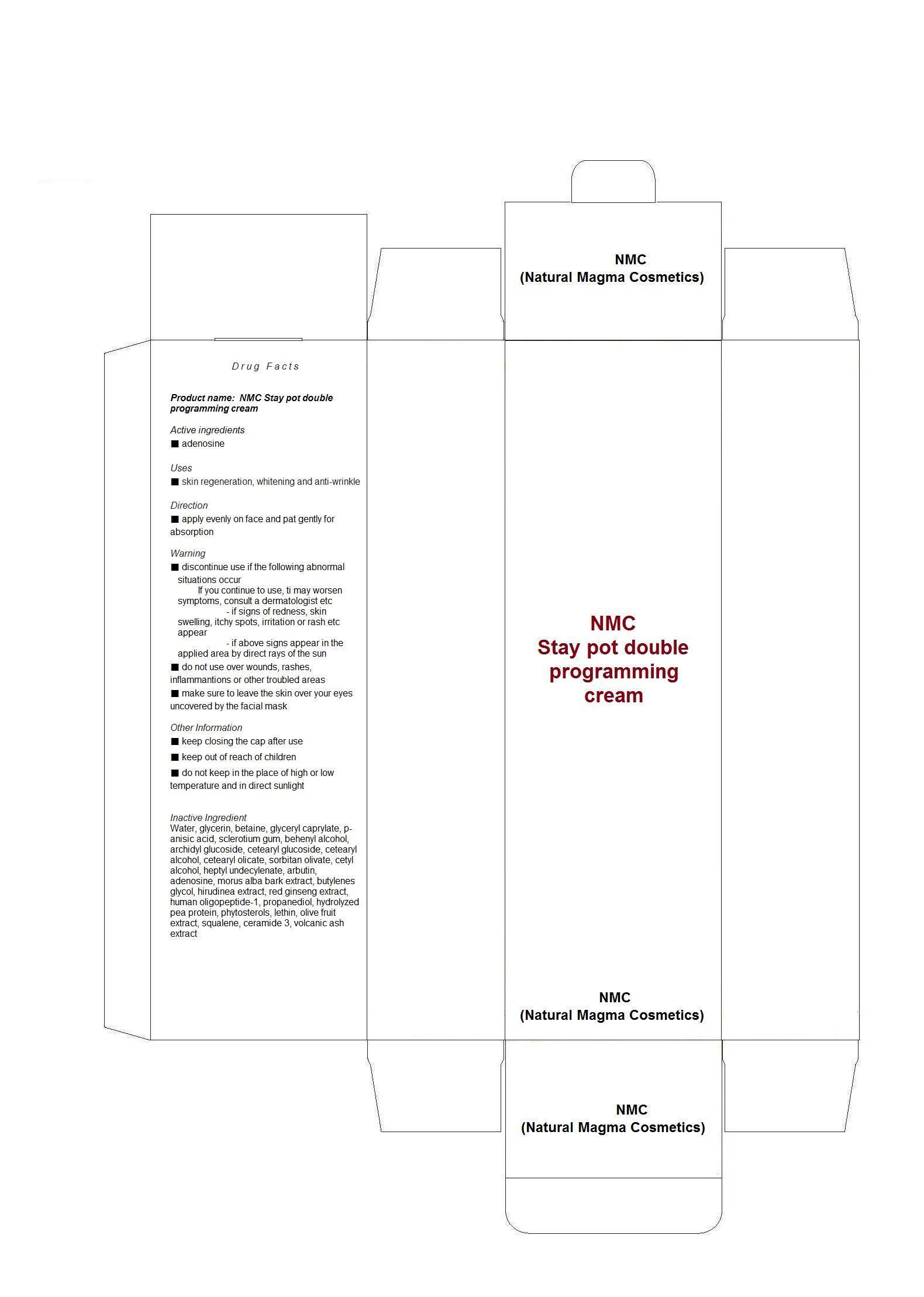
Water, glycerin, betaine, glyceryl caprylate, p-anisic acid, sclerotium gum, behenyl alcohol, archidyl glucoside, cetearyl glucoside, cetearyl alcohol, cetearyl olicate, sorbitan olivate, cetyl alcohol, heptyl undecylenate, arbutin, adenosine, morus alba bark extract, butylenes glycol, hirudinea extract, red ginseng extract, human oligopeptide-1, propanediol, hydrolyzed pea protein, phytosterols, lethin, olive fruit extract, squalene, ceramide 3, volcanic ash extract
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
■ discontinue use if the following abnormal situations occur
If you continue to use, ti may worsen symptoms, consult a dermatologist etc
- if signs of redness, skin swelling, itchy spots, irritation or rash etc appear
- if above signs appear in the applied area by direct rays of the sun
■ do not use over wounds, rashes, inflammantions or other troubled areas
■ make sure to leave the skin over your eyes uncovered by the facial mask
Other Information
■ keep closing the cap after use
■ keep out of reach of children
■ do not keep in the place of high or low temperature and in direct sunlight - DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NMC MAGIC SERUM
adenosine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59220-3001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ADENOSINE (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) ADENOSINE 0.04 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) BETAINE (UNII: 3SCV180C9W) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) P-ANISIC ACID (UNII: 4SB6Y7DMM3) DOCOSANOL (UNII: 9G1OE216XY) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) SORBITAN OLIVATE (UNII: MDL271E3GR) SQUALENE (UNII: 7QWM220FJH) PROPANEDIOL (UNII: 5965N8W85T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59220-3001-1 45 mL in 1 BOTTLE, WITH APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/11/2013 Labeler - NMC (Natural Magma Cosmetics) (688411932) Registrant - NMC (Natural Magma Cosmetics) (688411932) Establishment Name Address ID/FEI Business Operations NMC (Natural Magma Cosmetics) 688411932 manufacture(59220-3001)