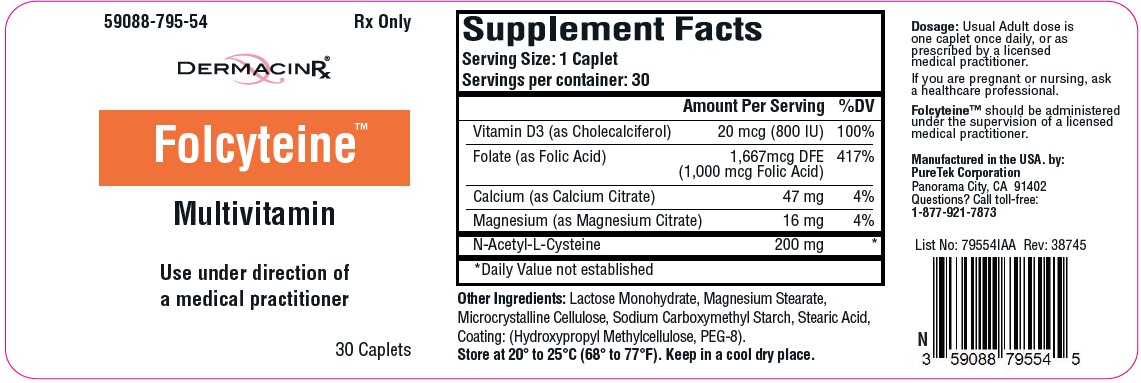
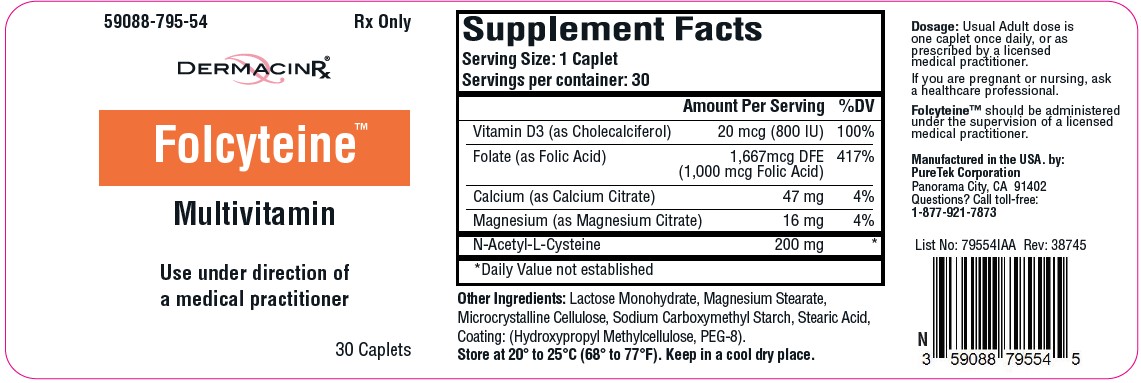
Label: FOLCYTEINE- multivitamin tablet
- NDC Code(s): 59088-795-54
- Packager: PureTek Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
Each caplet contains:
Vitamin D3 (as Cholecalciferol) .................. 20 mcg (800 IU)
Folate (as Folic Acid)...1,667 mcg DFE (1,000 mcg Folic Acid)
Calcium (as Calcium Citrate)....................................... 47 mg
Magnesium (as Magnesium Citrate)........................... 16 mg
N-Acetyl-L-Cysteine................................................... 200 mg - Other Ingredients:
- Indications and Usage:
- Contraindications:
-
BOXED WARNING
(What is this?)
Warnings:
Tell your doctor if you have: kidney problems, thyroid disease. This medication should be used as directed during pregnancy or while breast-feeding. Consult your doctor. Administration of folic acid alone is improper therapy for pernicious anemia and other megaloblastic anemias in which vitamin B12 is deficient.
Precautions
Folic acid in doses above 0.1 mg daily may obscure pernicious anemia, in that hematologic remission can occur while neurological manifestations remain progressive. There is a potential danger in administering folic acid to patients with undiagnosed anemia, since folic acid may obscure the diagnosis of pernicious anemia by alleviating the hematologic manifestations of the disease while allowing the neurologic complications to progress. This may result in severe nervous system damage before the correct diagnosis is made. The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
For use on the order of a healthcare practitioner.
Call your doctor about side effects. To report side effects, call PureTek Corporation at 1-877-921-7873 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
- Adverse Reactions:
- Dosage and Administration:
- How Supplied:
-
Storage
Do not use if bottle seal is broken.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Store at controlled room temperature 20° to 25°C (68° to 77°F). [See USP]. Protect from light and moisture and avoid excessive heat.
To report a serious adverse event or to obtain product information, contact 877-921-7873.
Manufactured by:
PureTek Corporation Panorama City, CA 91402
For questions or information call toll-free: 877-921-7873
- Folcyteine™
-
INGREDIENTS AND APPEARANCE
FOLCYTEINE
multivitamin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59088-795 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CITRATE (UNII: MLM29U2X85) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CITRATE 47 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 800 [iU] FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1000 ug ACETYLCYSTEINE (UNII: WYQ7N0BPYC) (ACETYLCYSTEINE - UNII:WYQ7N0BPYC) ACETYLCYSTEINE 200 mg MAGNESIUM CITRATE (UNII: RHO26O1T9V) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CITRATE 16 mg Inactive Ingredients Ingredient Name Strength STEARIC ACID (UNII: 4ELV7Z65AP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) Product Characteristics Color yellow (Pale Yellow) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59088-795-54 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/14/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/14/2023 Labeler - PureTek Corporation (785961046)