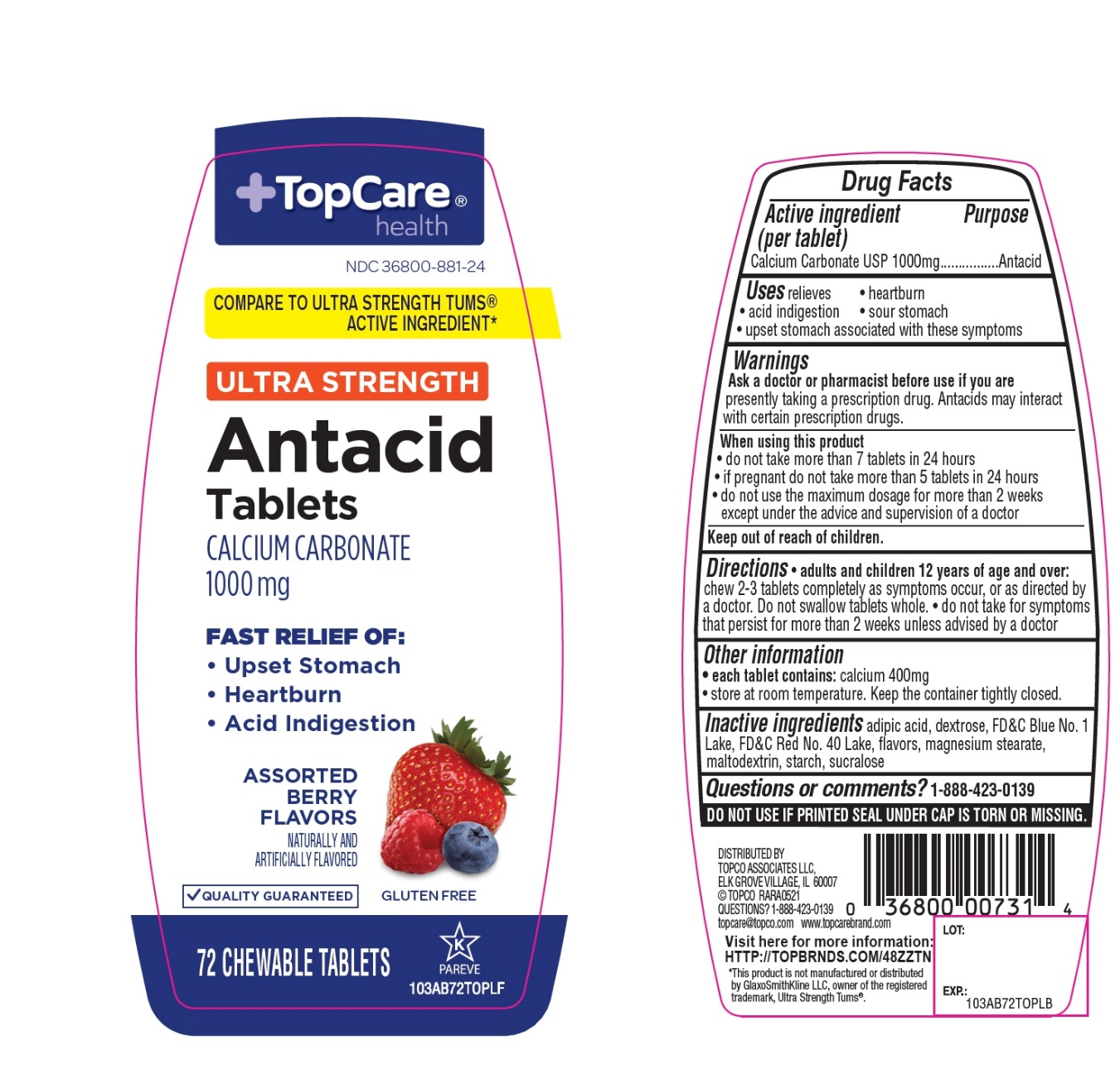
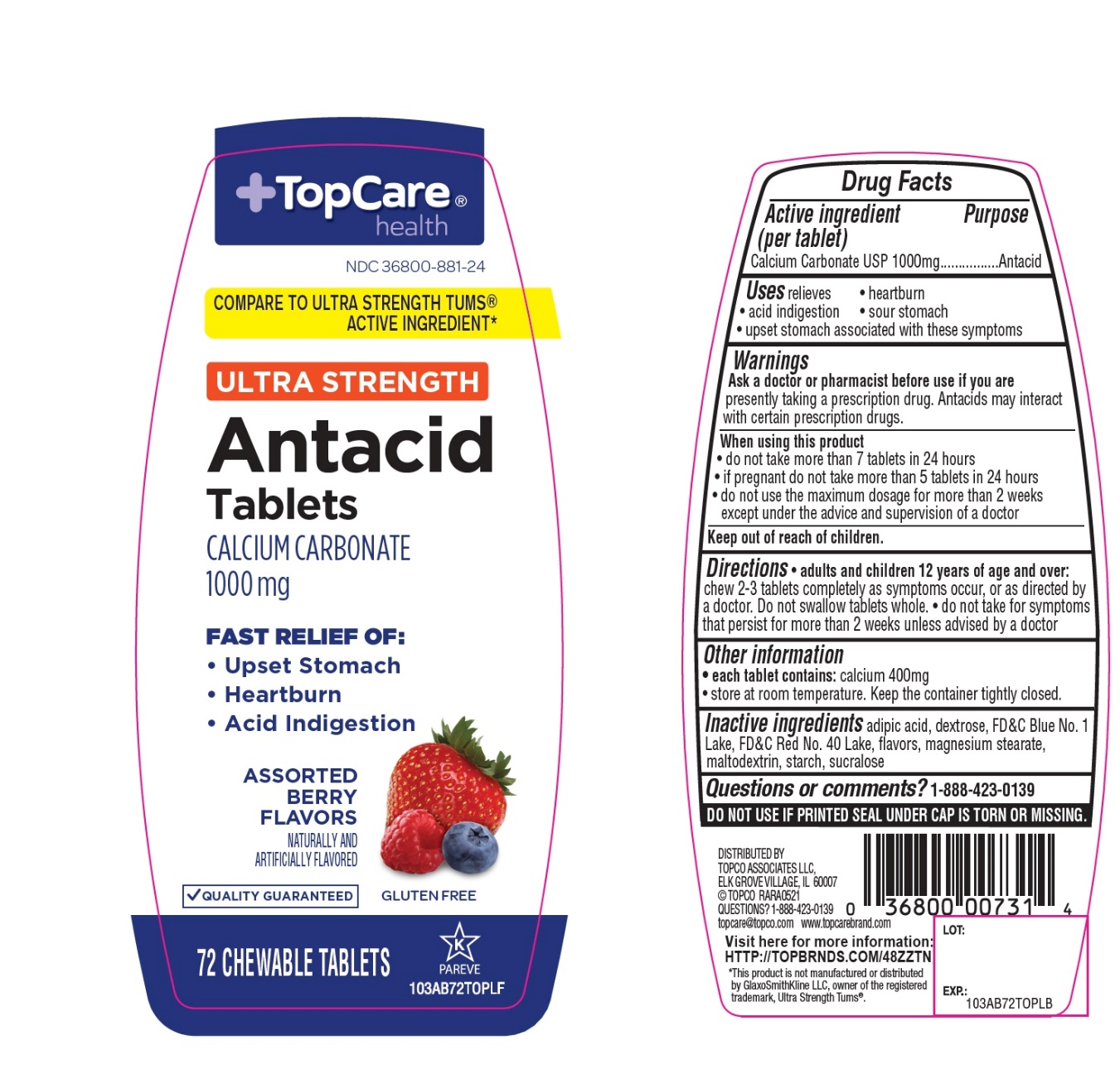
Label: TOPCARE ANTACID CALCIUM- calcium carbonate tablet, chewable
- NDC Code(s): 36800-881-24
- Packager: Topco Associates LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (per tablet)
- Purpose
- Uses
- Warnings
- Ask a doctor or pharmacist before use if you are
- When using this product
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions?
-
Package/Label Principal Display Panel
+TopCare® health TM
NDC# 36800-881-24
COMPARE TO ULTRA STRENGTH TUMS® ACTIVE INGREDIENT*
ULTRA STRENGTH
Antacid Tablets
CALCIUM CARBONATE 1000 mg
OUR PHARMACISTS RECOMMEND
FAST RELIEF OF:
- •
- Upset Stomach
- •
- Heartburn
- •
- Acid Indigestion
ASSORTED BERRY FLAVORS
QUALITY GUARANTEED
GLUTEN FREE
72 CHEWABLE TABLETS
K PAREVE
DISTRIBUTED BY
TOPCO ASSOCIATED LLC
ELK GROVE VILLAGE, IL 60007
©TOPCO RARA0918
QUESTIONS? 1-888-423-0139
topcare@topco.com www.topcarebrand.com
Visit here for more information: http://topbrnds.com/48ZS23
*This product is not manufactured or distributed by GlaxoSmithKline LLC, owner of the registered trademark, Ultra Strength Tums®.
-
INGREDIENTS AND APPEARANCE
TOPCARE ANTACID CALCIUM
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:36800-881 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB, CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 1000 mg Inactive Ingredients Ingredient Name Strength ADIPIC ACID (UNII: 76A0JE0FKJ) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) STARCH, CORN (UNII: O8232NY3SJ) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color RED, PINK, PURPLE Score no score Shape ROUND Size 19mm Flavor BERRY (assorted berry) Imprint Code L881 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:36800-881-24 72 in 1 BOTTLE; Type 0: Not a Combination Product 12/15/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 12/15/2018 Labeler - Topco Associates LLC (006935977)