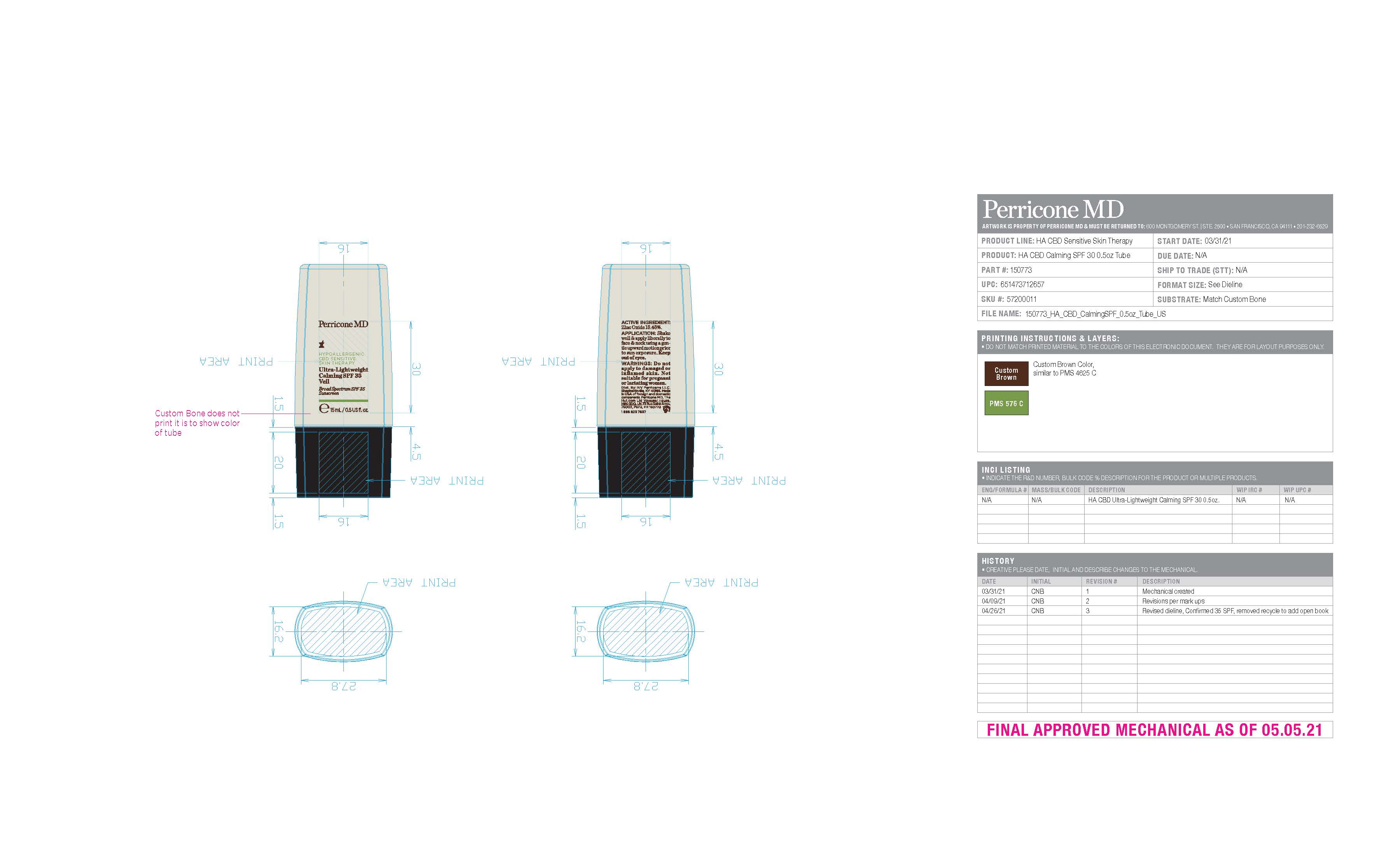
Label: HYPOALLERGENIC CBD SENSITIVE SKIN THERAPY ULTRA-LIGHTWEIGHT CALMING VEIL SPF 35- zinc oxide cream
- NDC Code(s): 45634-716-15
- Packager: NV Perricone LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- WARNINGS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HYPOALLERGENIC CBD SENSITIVE SKIN THERAPY ULTRA-LIGHTWEIGHT CALMING VEIL SPF 35
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:45634-716 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.3175 g in 15 g Inactive Ingredients Ingredient Name Strength CANNABIDIOL (UNII: 19GBJ60SN5) ROSEMARY (UNII: IJ67X351P9) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) LINGONBERRY (UNII: 0UNK9RZQ7X) CALCIUM GLUCONATE (UNII: SQE6VB453K) WATER (UNII: 059QF0KO0R) ISODODECANE (UNII: A8289P68Y2) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) C9-12 ALKANE (UNII: 7J5R5W72QM) PROPYLENE CARBONATE (UNII: 8D08K3S51E) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) GLYCERIN (UNII: PDC6A3C0OX) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) SODIUM BENZOATE (UNII: OJ245FE5EU) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) CASTOR OIL (UNII: D5340Y2I9G) RICE BRAN (UNII: R60QEP13IC) TRIHEPTANOIN (UNII: 2P6O7CFW5K) DILINOLEIC ACID/BUTANEDIOL COPOLYMER (UNII: 1F2S8T535O) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) STEAROYL GLUTAMIC ACID (UNII: 4R4O71786G) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) XANTHAN GUM (UNII: TTV12P4NEE) GLUCONOLACTONE (UNII: WQ29KQ9POT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPANEDIOL (UNII: 5965N8W85T) SODIUM CHLORIDE (UNII: 451W47IQ8X) LEVOMENOL (UNII: 24WE03BX2T) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45634-716-15 9.3 g in 1 TUBE; Type 0: Not a Combination Product 09/06/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 09/05/2023 Labeler - NV Perricone LLC (054414243) Establishment Name Address ID/FEI Business Operations Dimensional Merchandising Inc (DMI) 076693183 manufacture(45634-716)