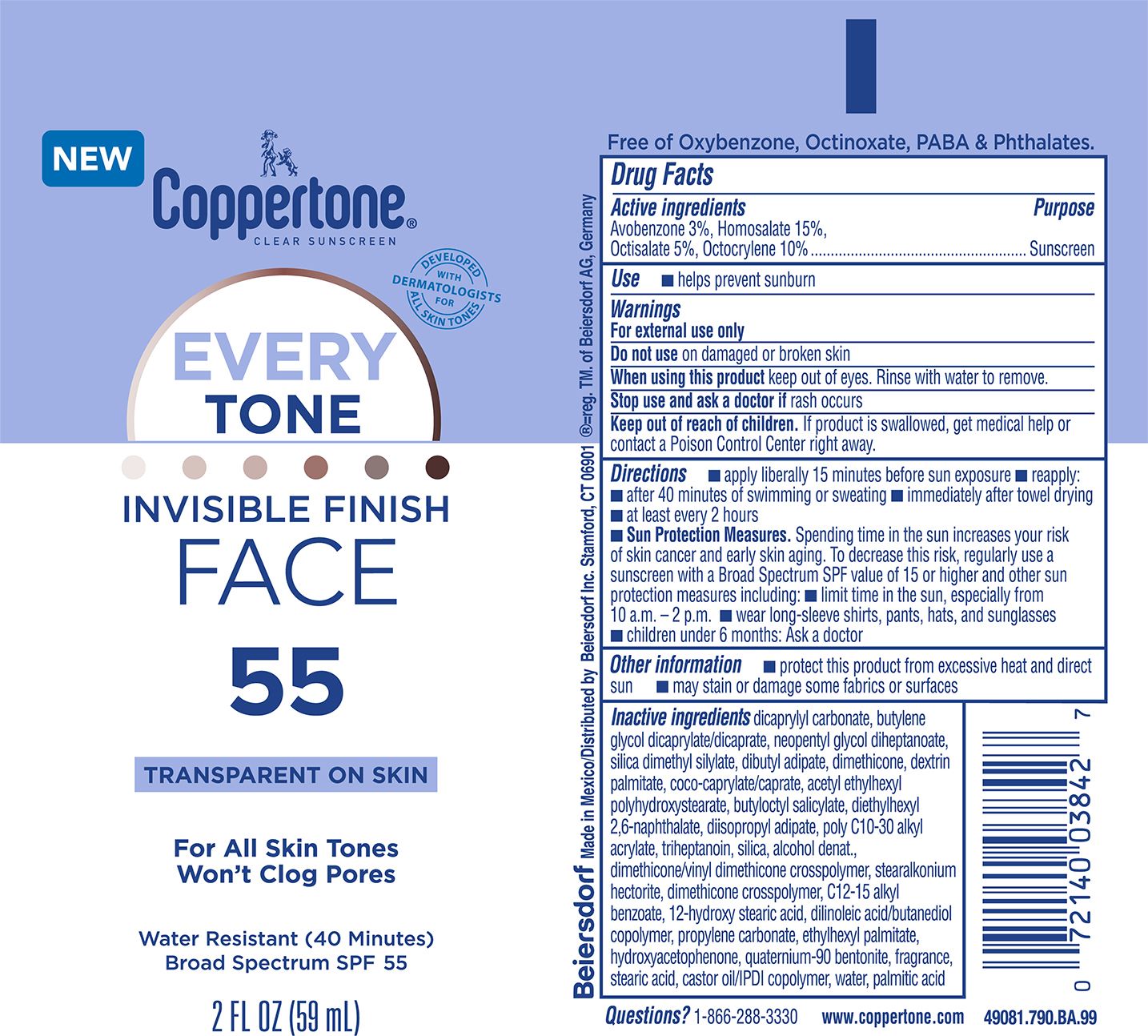
Label: COPPERTONE EVERYTONE FACE SUNSCREEN SPF 55- avobenzone 3%, homosalate 15%, octisalate 5%, octocrylene 10% lotion
- NDC Code(s): 66800-1094-2
- Packager: Beiersdorf Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drugs Facts
- Active ingredients
- Purpose
- Use
- Warnings
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
■ apply liberally 15 minutes before sun exposure
■ reapply:
■ after 40 minutes of swimming or sweating
■ immediately after towel drying
■ at least every 2 hours
■ Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
■ limit time in the sun, especially from 10 a.m. – 2 p.m.
■ wear long-sleeve shirts, pants, hats, and sunglasses
■ children under 6 months: Ask a doctor
- Other information
-
Inactive ingredients
dicaprylyl carbonate, butylene glycol dicaprylate/dicaprate, neopentyl glycol diheptanoate, silica dimethyl silylate, dibutyl adipate, dimethicone, dextrin palmitate, coco-caprylate/caprate, acetyl ethylhexyl polyhydroxystearate, butyloctyl salicylate, diethylhexyl 2,6-naphthalate, diisopropyl adipate, poly C10-30 alkyl acrylate, triheptanoin, silica, alcohol denat., dimethicone/vinyl dimethicone crosspolymer, stearalkonium hectorite, dimethicone crosspolymer, C12-15 alkyl benzoate, 12-hydroxy stearic acid, dilinoleic acid/butanediol copolymer, propylene carbonate, ethylhexyl palmitate, hydroxyacetophenone, quaternium-90 bentonite, fragrance, stearic acid, castor oil/IPDI copolymer, water, palmitic acid
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COPPERTONE EVERYTONE FACE SUNSCREEN SPF 55
avobenzone 3%, homosalate 15%, octisalate 5%, octocrylene 10% lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66800-1094 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 15 g in 100 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 10 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 g Inactive Ingredients Ingredient Name Strength PROPYLENE CARBONATE (UNII: 8D08K3S51E) PALMITIC ACID (UNII: 2V16EO95H1) DIETHYLHEXYL 2,6-NAPHTHALATE (UNII: I0DQJ7YGXM) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) TRIHEPTANOIN (UNII: 2P6O7CFW5K) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) 12-HYDROXYSTEARIC ACID (UNII: 933ANU3H2S) ETHYLHEXYL PALMITATE (UNII: 2865993309) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) ISOPHORONE DIISOCYANATE (UNII: 43B0856528) STEARIC ACID (UNII: 4ELV7Z65AP) DILINOLEIC ACID/BUTANEDIOL COPOLYMER (UNII: 1F2S8T535O) WATER (UNII: 059QF0KO0R) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) DEXTRIN PALMITATE (CORN; 20000 MW) (UNII: 89B2BSF9I3) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) FRAGRANCE CLEAN ORC0600327 (UNII: 329LCV5BTF) ALCOHOL (UNII: 3K9958V90M) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIMETHICONE (UNII: 92RU3N3Y1O) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) DIBUTYL ADIPATE (UNII: F4K100DXP3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color white (white to off-white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66800-1094-2 59 g in 1 TUBE; Type 0: Not a Combination Product 11/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2023 Labeler - Beiersdorf Inc (001177906)