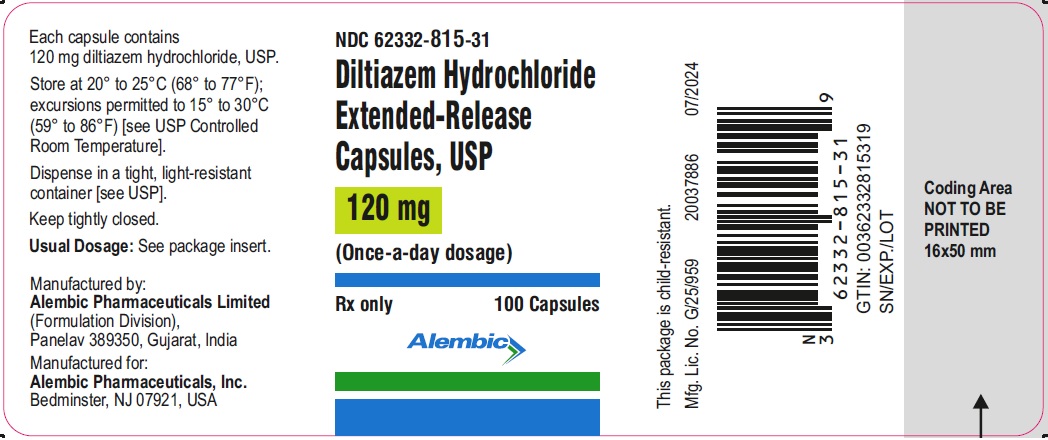
Label: DILTIAZEM HYDROCHLORIDE capsule, extended release
-
NDC Code(s):
62332-815-31,
62332-815-71,
62332-815-91,
62332-816-31, view more62332-816-71, 62332-816-91, 62332-817-31, 62332-817-71, 62332-817-91
- Packager: Alembic Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
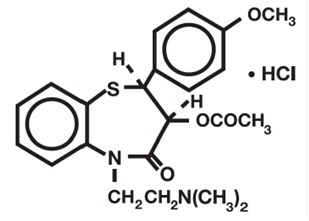
Diltiazem hydrochloride, USP is a calcium ion influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride, USP is 1,5-Benzothiazepin-4(5H)one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-, monohydrochloride, (+)-cis-. Its molecular formula is C22H26N2O4S•HCl and its molecular weight is 450.98. Its structural formula is as follows:
Diltiazem hydrochloride, USP is a white to off-white crystalline powder with a bitter taste. It is freely soluble in chloroform, formic acid, methanol and water, sparingly soluble in dehydrated ethanol and insoluble in ether.
Diltiazem hydrochloride extended-release capsules, USP contain 120 mg or 180 mg or 240 mg diltiazem hydrochloride and are formulated for the controlled release of diltiazem hydrochloride over a 24-hour period.
Inactive Ingredients: Diltiazem hydrochloride extended-release capsules, USP contain lactose monohydrate, hypromellose, colloidal silicon dioxide and magnesium stearate.
The 120 mg, 180 mg and 240 mg capsule shells contain FD&C Blue 1, FD&C Red 3, FD&C Red 40, titanium dioxide and gelatin.
Additionally, 120 mg capsule shell contains FD&C Yellow 6, 180 mg and 240 mg capsule shell contains D&C Red 33.
The capsules are printed with edible ink containing shellac, iron oxide black and potassium hydroxide.
For oral administration.
FDA approved dissolution test specifications differ from USP.
-
CLINICAL PHARMACOLOGY
The therapeutic benefits of diltiazem hydrochloride are believed to be related to its ability to inhibit the influx of calcium ions during membrane depolarization of cardiac and vascular smooth muscles.
Mechanism of Action
Hypertension
Diltiazem hydrochloride extended-release capsule produces its antihypertensive effect primarily by relaxation of vascular smooth muscle with a resultant decrease in peripheral vascular resistance. The magnitude of blood pressure reduction is related to the degree of hypertension; thus hypertensive individuals experience an antihypertensive effect, whereas there is only a modest fall in blood pressure in normotensives.
Angina
Diltiazem HCl has been shown to produce increases in exercise tolerance, probably due to its ability to reduce myocardial oxygen demand. This is accomplished via reductions in heart rate and systemic blood pressure at submaximal and maximal work loads.
Diltiazem has been shown to be a potent dilator of coronary arteries, both epicardial and subendocardial. Spontaneous and ergonovine-induced coronary artery spasms are inhibited by diltiazem.
In animal models, diltiazem interferes with the slow inward (depolarizing) current in excitable tissue. It causes excitation-contraction uncoupling in various myocardial tissues without changes in the configuration of the action potential. Diltiazem produces relaxation of coronary vascular smooth muscle and dilation of both large and small coronary arteries at drug levels which cause little or no negative inotropic effect. The resultant increases in coronary blood flow (epicardial and subendocardial) occur in ischemic and nonischemic models and are accompanied by dose-dependent decreases in systemic blood pressure and decreases in peripheral resistance.
Hemodynamic and Electrophysiologic Effects
Like other calcium antagonists, diltiazem decreases sinoatrial and atrioventricular conduction in isolated tissues and has a negative inotropic effect in isolated preparations. In the intact animal, prolongation of the AH interval can be seen at higher doses.
In man, diltiazem prevents spontaneous and ergonovine-provoked coronary artery spasm. It causes a decrease in peripheral vascular resistance and a modest fall in blood pressure in normotensive individuals. In exercise tolerance studies in patients with ischemic heart disease, diltiazem reduces the double product (HR x SBP) for any given work load. Studies to date, primarily in patients with good ventricular function, have not revealed evidence of a negative inotropic effect. Cardiac output, ejection fraction and left ventricular end diastolic pressure have not been affected. Such data have no predictive value with respect to effects in patients with poor ventricular function. Increased heart failure has, however, been reported in occasional patients with pre-existing impairment of ventricular function. There are as yet few data on the interaction of diltiazem and beta-blockers in patients with poor ventricular function. Resting heart rate is usually slightly reduced by diltiazem.
Diltiazem hydrochloride extended-release capsule produces antihypertensive effects both in the supine and standing positions. Postural hypotension is infrequently noted upon suddenly assuming an upright position. Diltiazem decreases vascular resistance, increases cardiac output (by increasing stroke volume), and produces a slight decrease or no change in heart rate. No reflex tachycardia is associated with the chronic antihypertensive effects.
During dynamic exercise, increases in diastolic pressure are inhibited while maximum achievable systolic pressure is usually reduced. Heart rate at maximum exercise does not change or is slightly reduced.
Diltiazem antagonizes the renal and peripheral effects of angiotensin II. No increased activity of the renin-angiotensin-aldosterone axis has been observed. Chronic therapy with diltiazem produces no change or an increase in plasma catecholamines. Hypertensive animal models respond to diltiazem with reductions in blood pressure and increased urinary output and natriuresis without a change in the urinary sodium/potassium ratio. In man, transient natriuresis and kaliuresis have been reported, but only in high intravenous doses of 0.5 mg/kg of body weight.
Diltiazem-associated prolongation of the AH interval is not more pronounced in patients with first-degree heart block. In patients with sick sinus syndrome, diltiazem significantly prolongs sinus cycle length (up to 50% in some cases). Intravenous diltiazem in doses of 20 mg prolongs AH conduction time and AV node functional and effective refractory periods approximately 20%.
In two short-term, double-blind, placebo-controlled studies, 303 hypertensive patients were treated with once daily diltiazem hydrochloride extended-release capsules in doses of up to 540 mg. There were no instances of greater than first-degree atrioventricular block, and the maximum increase in the PR interval was 0.08 seconds. No patients were prematurely discontinued from the medication due to symptoms related to prolongation of the PR interval.
Pharmacodynamics
In one short-term, double-blind, placebo-controlled study, diltiazem hydrochloride extended-release capsule 120, 240, 360, and 480 mg/day demonstrated a dose-related antihypertensive response among patients with mild to moderate hypertension. Statistically significant decreases in trough mean supine diastolic blood pressure were seen through 4 weeks of treatment: 120 mg/day (-5.1 mmHg); 240 mg/day (-6.9 mmHg); 360 mg/day (-6.9 mmHg); and, 480 mg/day (-10.6 mmHg). Statistically significant decreases in trough mean supine systolic blood pressure were also seen through 4 weeks of treatment: 120 mg/day (-2.6 mmHg); 240 mg/day (-6.5 mmHg); 360 mg/day (-4.8 mmHg); and 480 mg/day (-10.6 mmHg). The proportion of evaluable patients exhibiting a therapeutic response (supine diastolic blood pressure <90 mmHg or decrease >10 mmHg) was greater as the dose increased: 31%, 42%, 48%, and 69% with the 120, 240, 360, and 480 mg/day diltiazem groups, respectively. Similar findings were observed for standing systolic and diastolic blood pressures. The trough (24 hours after a dose) antihypertensive effect of diltiazem hydrochloride extended-release capsules retained more than one-half of the response seen at peak (3 to 6 hours after administration).
Significant reductions of mean supine blood pressure (at trough) in patients with mild to moderate hypertension were also seen in a short-term, double-blind, dose-escalation, placebo-controlled study after 2 weeks of once daily diltiazem hydrochloride extended-release capsules 180 mg/day (diastolic: -6.1 mmHg; systolic: -4.7 mmHg) and again, 2 weeks after escalation to 360 mg/day (diastolic: -9.3 mmHg; systolic: -7.2 mmHg). However, a further increase in dose to 540 mg/day for 2 weeks provided only a minimal further increase in the antihypertensive effect (diastolic: -10.2 mmHg; systolic: -6.7 mmHg).
Diltiazem hydrochloride extended-release capsules, given at 120 mg, 240 mg, and 480 mg/day, in a randomized, multicenter, double-blind, placebo-controlled, parallel group, dose-ranging study, in 189 patients with chronic angina, demonstrated a dose-related increase in exercise time by Exercise Tolerance Test (ETT) and a reduction in rates of anginal attacks (based on individual patient diaries). The improvement in total exercise time (using the Bruce protocol), measured at trough exercise periods, for placebo, 120 mg, 240 mg, and 480 mg, was 20, 37, 49, and 56 seconds, respectively.
Pharmacokinetics and Metabolism
Diltiazem is well absorbed from the gastrointestinal tract, and is subject to an extensive first-pass effect. When given as an immediate release oral formulation, the absolute bioavailability (compared to intravenous administration) of diltiazem is approximately 40%. Diltiazem undergoes extensive hepatic metabolism in which 2% to 4% of the unchanged drug appears in the urine. Total radioactivity measurement following short IV administration in healthy volunteers suggests the presence of other unidentified metabolites which attain higher concentrations than those of diltiazem and are more slowly eliminated; half-life of total radioactivity is about 20 hours compared to 2 to 5 hours for diltiazem. In vitro binding studies show diltiazem HCl is 70% to 80% bound to plasma proteins. Competitive in vitro ligand binding studies have also shown diltiazem HCl binding is not altered by therapeutic concentrations of digoxin, HCTZ, phenylbutazone, propranolol, salicylic acid, or warfarin. The plasma elimination half-life of diltiazem is approximately 3.0 to 4.5 hours. Desacetyldiltiazem, the major metabolite of diltiazem, which is also present in the plasma at concentrations of 10% to 20% of the parent drug, is approximately 25% to 50% as potent a coronary vasodilator as diltiazem. Therapeutic blood levels of diltiazem hydrochloride appear to be in the range of 40 to 200 ng/mL. There is a departure from linearity when dose strengths are increased; the half-life is slightly increased with dose.
A study that compared patients with normal hepatic function to patients with cirrhosis found an increase in half-life and a 69% increase in bioavailability in the hepatically impaired patients. Patients with severely impaired renal function showed no difference in the pharmacokinetic profile of diltiazem compared to patients with normal renal function.
Diltiazem hydrochloride extended-release capsules (Once-a-day dosage) contain a degradable controlled-release tablet formulation designed to release diltiazem over a 24-hour period. Controlled absorption of diltiazem begins within 1 hour, with maximum plasma concentrations being achieved 4 to 6 hours after administration. The apparent steady-state half-life of diltiazem following once daily administration of diltiazem hydrochloride extended-release capsules ranges from 5 to 10 hours. This prolongation of half-life is attributed to continued absorption of diltiazem rather than to alterations in its elimination.
The absolute bioavailability of diltiazem from a single dose of diltiazem hydrochloride extended-release capsules (compared to intravenous administration) is 41% (±14). The value was shown to be similar to the 40% systemic availability reported following administration of an immediate release diltiazem HCl formulation.
As the dose of diltiazem hydrochloride extended-release capsule is increased from a daily dose of 120 mg to 240 mg, there is an increase in the AUC of 2.3 fold. When the dose is increased from 240 mg to 360 mg, AUC increases 1.6 fold and when increased from 240 mg to 480 mg, AUC increases 2.4 fold.
In vivo release of diltiazem occurs throughout the gastrointestinal tract, with controlled release still occurring for up to 24 hours after administration, as determined by radio-labeled methods. As the once daily dose of diltiazem hydrochloride extended-release capsule was increased, departures from linearity were noted. There were disproportionate increases in area under the curve for doses from 120 mg to 480 mg.
The presence of food did not affect the ability of diltiazem hydrochloride extended-release capsules to maintain a controlled release of the drug and did not impact its sustained release properties over 24-hours after administration. However, simultaneous administration of diltiazem hydrochloride extended-release capsules with a high-fat breakfast resulted in increases in AUC of 13% and 19%, and in Cmax by 37% and 51%, respectively.
-
INDICATIONS AND USAGE
Diltiazem hydrochloride extended-release capsules are indicated for the treatment of hypertension. Diltiazem hydrochloride may be used alone or in combination with other antihypertensive medications, such as diuretics.
Diltiazem hydrochloride extended-release capsules are indicated for the management of chronic stable angina.
-
CONTRAINDICATIONS
Diltiazem hydrochloride is contraindicated in: (1) patients with sick sinus syndrome except in the presence of a functioning ventricular pacemaker; (2) patients with second or third degree AV block except in the presence of a functioning ventricular pacemaker; (3) patients with hypotension (less than 90 mmHg systolic); (4) patients who have demonstrated hypersensitivity to the drug; and (5) patients with acute myocardial infarction and pulmonary congestion as documented by X-ray on admission.
-
WARNINGS
Cardiac Conduction
Diltiazem hydrochloride prolongs AV node refractory periods without significantly prolonging sinus node recovery time, except in patients with sick sinus syndrome. This effect may rarely result in abnormally slow heart rates (particularly in patients with sick sinus syndrome) or second, or third degree AV block (22 of 10,119 patients, or 0.2%); 41% of these 22 patients were receiving concomitant β-adrenoceptor antagonists versus 17% of the total group. Concomitant use of diltiazem with beta-blockers or digitalis may result in additive effects on cardiac conduction. A patient with Prinzmetal’s angina developed periods of asystole (2 to 5 seconds) after a single 60 mg dose of diltiazem.
Congestive Heart Failure
Although diltiazem has a negative inotropic effect in isolated animal tissue preparations, hemodynamic studies in humans with normal ventricular function have not shown a reduction in cardiac index nor consistent negative effects on contractility (dp/dt). An acute study of oral diltiazem in patients with impaired ventricular function (ejection fraction of 24% ± 6%) showed improvement in indices of ventricular function without significant decrease in contractile function (dp/dt). Worsening of congestive heart failure has been reported in patients with preexisting impairment of ventricular function. Experience with the use of diltiazem hydrochloride in combination with beta-blockers in patients with impaired ventricular function is limited. Caution should be exercised when using this combination.
Hypotension
Decreases in blood pressure associated with diltiazem hydrochloride therapy may occasionally result in symptomatic hypotension.
Acute Hepatic Injury
Mild elevations of serum transaminases with and without concomitant elevation in alkaline phosphatase and bilirubin have been observed in clinical studies. Such elevations were usually transient and frequently resolved even with continued diltiazem treatment. In rare instances, significant elevations in alkaline phosphatase, LDH, SGOT, SGPT, and other phenomena consistent with acute hepatic injury have been noted. These reactions tended to occur early after therapy initiation (1 to 6 weeks) and have been reversible upon discontinuation of drug therapy. The relationship to diltiazem is uncertain in some cases, but probable in some others. (See PRECAUTIONS.) -
PRECAUTIONS
General
Diltiazem hydrochloride is extensively metabolized by the liver and is excreted by the kidneys and in bile. As with any drug given over prolonged periods, laboratory parameters should be monitored at regular intervals. The drug should be used with caution in patients with impaired renal or hepatic function. In subacute and chronic dog and rat studies designed to produce toxicity, high doses of diltiazem were associated with hepatic damage. In special subacute hepatic studies, oral doses of 125 mg/kg and higher in rats were associated with histological changes in the liver which were reversible when the drug was discontinued. In dogs, doses of 20 mg/kg were also associated with hepatic changes; however, these changes were reversible with continued dosing.
Dermatological events (see ADVERSE REACTIONS) may be transient and may disappear despite continued use of diltiazem hydrochloride. However, skin eruptions progressing to erythema multiforme and/or exfoliative dermatitis have also been infrequently reported. Should a dermatologic reaction persist, the drug should be discontinued.
Although diltiazem hydrochloride extended-release capsule utilizes a slowly disintegrating matrix, caution should still be used in patients with preexisting severe gastrointestinal narrowing (pathologic or iatrogenic). There have been no reports of obstructive symptoms in patients with known strictures in association with the ingestion of diltiazem hydrochloride extended-release capsules.
Information for Patients
Diltiazem hydrochloride extended-release capsules should be taken on an empty stomach. Patients should be cautioned that the diltiazem hydrochloride extended-release capsules should not be opened, chewed or crushed, and should be swallowed whole.
Drug Interactions
Due to the potential for additive effects, caution and careful titration are warranted in patients receiving diltiazem hydrochloride concomitantly with any agents known to affect cardiac contractility and/or conduction. (See WARNINGS.) Pharmacologic studies indicate that there may be additive effects in prolonging AV conduction when using beta-blockers or digitalis concomitantly with diltiazem hydrochloride. (See WARNINGS.) As with all drugs, care should be exercised when treating patients with multiple medications. Diltiazem hydrochloride undergoes biotransformation by cytochrome P-450 mixed function oxidase. Co-administration of diltiazem hydrochloride with other agents which follow the same route of biotransformation may result in the competitive inhibition of metabolism. Especially in patients with renal and/or hepatic impairment, dosages of similarly metabolized drugs, particularly those of low therapeutic ratio such as cyclosporine, may require adjustment when starting or stopping concomitantly administered diltiazem hydrochloride to maintain optimum therapeutic blood levels. Concomitant administration of diltiazem with carbamazepine has been reported to result in elevated plasma levels of carbamazepine, resulting in toxicity in some cases.
Beta-Blockers
Controlled and uncontrolled domestic studies suggest that concomitant use of diltiazem hydrochloride and beta-blockers is usually well-tolerated, but available data are not sufficient to predict the effects of concomitant treatment in patients with left ventricular dysfunction or cardiac conduction abnormalities. Administration of diltiazem hydrochloride concomitantly with propranolol in five normal volunteers resulted in increased propranolol levels in all subjects and the bioavailability of propranolol was increased approximately 50%. If combination therapy is initiated or withdrawn in conjunction with propranolol, an adjustment in the propranolol dose may be warranted. (See WARNINGS.)
Cimetidine
A study in six healthy volunteers has shown a significant increase in peak diltiazem plasma levels (58%) and area-under-the-curve (53%) after a 1 week course of cimetidine at 1,200 mg per day and diltiazem 60 mg per day. Ranitidine produced smaller, nonsignificant increases. The effect may be mediated by cimetidine’s known inhibition of hepatic cytochrome P-450, the enzyme system responsible for the first-pass metabolism of diltiazem. Patients currently receiving diltiazem therapy should be carefully monitored for a change in pharmacological effect when initiating and discontinuing therapy with cimetidine. An adjustment in the diltiazem dose may be warranted.
Clonidine
Sinus bradycardia resulting in hospitalization and pacemaker insertion has been reported in association with the use of clonidine concurrently with diltiazem. Monitor heart rate in patients receiving concomitant diltiazem and clonidine.
Digitalis
Administration of diltiazem hydrochloride with digoxin in 24 healthy male subjects increased plasma digoxin concentrations approximately 20%. Another investigator found no increase in digoxin levels in 12 patients with coronary artery disease. Since there have been conflicting results regarding the effects of digoxin levels, it is recommended that digoxin levels be monitored when initiating, adjusting, and discontinuing diltiazem hydrochloride therapy to avoid possible over- or under-digitalization. (See WARNINGS.)
Anesthetics
The depression of cardiac contractility, conductivity, and automaticity as well as the vascular dilation associated with anesthetics may be potentiated by calcium channel blockers. When used concomitantly, anesthetics and calcium channel blockers should be titrated carefully.
Statins
Diltiazem is an inhibitor of CYP3A4 and has been shown to increase significantly the AUC of some statins. The risk of myopathy and rhabdomyolysis with statins metabolized by CYP3A4 may be increased with concomitant use of diltiazem. When possible, use a non-CYP3A4-metabolized statin with diltiazem; otherwise, dose adjustments for both diltiazem and the statin should be considered along with close monitoring for signs and symptoms of any statin related adverse events.
In a healthy volunteer cross-over study (N=10), co-administration of a single 20 mg dose of simvastatin at the end of a 14 day regimen with 120 mg twice daily diltiazem SR resulted in a 5-fold higher mean simvastatin AUC compared with simvastatin alone. High average steady-state exposures of diltiazem would result in a greater increase in simvastatin exposure. A daily dose of 480 mg of diltiazem would be expected to result in an 8-fold higher mean simvastatin AUC compared with simvastatin alone. If coadministration of simvastatin with diltiazem is required, limit the daily doses of simvastatin to 10 mg and diltiazem to 240 mg.
In a ten-subject randomized, open label, 4-way cross-over study, co-administration of diltiazem (120 mg twice daily diltiazem SR for 2 weeks) with a single 20 mg dose of lovastatin resulted in 3- to 4- fold higher mean lovastatin AUC and Cmax values compared with lovastatin alone. In the same study, there was no significant change in 20 mg single dose pravastatin AUC and Cmax during diltiazem coadministration.
Carcinogenesis, Mutagenesis, Impairment of Fertility
A 24-month study in rats and an 18-month study in mice showed no evidence of carcinogenicity. There was also no mutagenic response in vitro or in vivo in mammalian cell assays or in vitro in bacteria. No evidence of impaired fertility was observed in male or female rats at oral doses of up to 100 mg/kg/day.
Pregnancy
Reproduction studies have been conducted in mice, rats, and rabbits. Administration of doses ranging from 4 to 6 times (depending on species) the upper limit of the optimum dosage range in clinical trials (480 mg once daily or 8 mg/kg once daily for a 60 kg patient) has resulted in embryo and fetal lethality. These studies have revealed, in one species or another, a propensity to cause abnormalities of the skeleton, heart, retina, and tongue. Also observed were reductions in early individual pup weights and pup survival, prolonged delivery, and increased incidence of stillbirths.
There are no well-controlled studies in pregnant women; therefore, use diltiazem hydrochloride in pregnant women only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Diltiazem is excreted in human milk. One report suggests that concentrations in breast milk may approximate serum levels. If use of diltiazem hydrochloride is deemed essential, an alternate method of infant feeding should be instituted.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established. -
ADVERSE REACTIONS
Serious adverse reactions to diltiazem hydrochloride have been rare in studies with other formulations, as well as with diltiazem hydrochloride extended-release capsules. It should be recognized, however, that patients with impaired ventricular function and cardiac conduction abnormalities have usually been excluded from these studies.
Hypertension
The most common adverse events (frequency ≥1%) in placebo-controlled, clinical hypertension studies with diltiazem hydrochloride extended-release capsules using daily doses up to 540 mg, are listed in the table below with placebo-treated patients included for comparison.
MOST COMMON ADVERSE EVENTS IN DOUBLE-BLIND, PLACEBO-CONTROLLED HYPERTENSION TRIALS
Adverse Events
(COSTART Term)
Diltiazem Hydrochloride Extended-Release Capsules*
n=303
# pts (%)
Placebo
n=87
# pts (%)
rhinitis
29 (9.6)
7 (8)
headache
27 (8.9)
12 (13.8)
pharyngitis
17 (5.6)
4 (4.6)
constipation
11 (3.6)
2 (2.3)
cough increase
9 (3)
2 (2.3)
flu syndrome
7 (2.3)
1 (1.1)
edema, peripheral
7 (2.3)
0 (0)
myalgia
7 (2.3)
0 (0)
diarrhea
6 (2)
0 (0)
vomiting
6 (2)
0 (0)
sinusitis
6 (2)
1 (1.1)
asthenia
5 (1.7)
0 (0)
pain, back
5 (1.7)
2 (2.3)
nausea
5 (1.7)
1 (1.1)
dyspepsia
4 (1.3)
0 (0)
vasodilatation
4 (1.3)
0 (0)
injury, accident
4 (1.3)
0 (0)
pain, abdominal
3 (1)
0 (0)
arthrosis
3 (1)
0 (0)
insomnia
3 (1)
0 (0)
dyspnea
3 (1)
0 (0)
rash
3 (1)
1 (1.1)
tinnitus
3 (1)
0 (0)
*Adverse events occurring in 1% or more of patients receiving diltiazem hydrochloride extended-release capsules.
Angina
The most common adverse events (frequency ≥1%) in a placebo-controlled, short-term (2 week) clinical angina study with diltiazem hydrochloride extended-release capsules are listed in the table below with placebo-treated patients included for comparison. In this trial, following a placebo phase, patients were randomly assigned to once daily doses of either 120, 240, or 480 mg of diltiazem hydrochloride extended-release capsules.
MOST COMMON ADVERSE EVENTS IN A DOUBLE-BLIND, PLACEBO-CONTROLLED SHORT-TERM, ANGINA TRIALS
Adverse Events
(COSTART Term)
Diltiazem Hydrochloride Extended-Release Capsules*
n=139
# pts (%)
Placebo
n=50
# pts (%)
asthenia
5 (3.6)
2 (4)
headache
4 (2.9)
3 (6)
pain, back
4 (2.9)
1 (2)
rhinitis
4 (2.9)
1 (2)
constipation
3 (2.2)
1 (2)
nausea
3 (2.2)
0 (0)
edema, peripheral
3 (2.2)
1 (2)
dizziness
3 (2.2)
0 (0)
cough, increased
3 (2.2)
0 (0)
bradycardia
2 (1.4)
0 (0)
fibrillation, atrial
2 (1.4)
0 (0)
arthralgia
2 (1.4)
0 (0)
dream, abnormal
2 (1.4)
0 (0)
dyspnea
2 (1.4)
0 (0)
pharyngitis
2 (1.4)
1 (2)
*Adverse events occurring in 1% or more of patients receiving diltiazem hydrochloride extended-release capsules.
Infrequent Adverse Events
The following additional events (COSTART Terms), listed by body system, were reported infrequently (less than 1%) in all subjects, hypertensive (n=425) or angina (n=318) patients who received diltiazem hydrochloride extended-release capsules, or with other formulations of diltiazem.
Hypertension
Cardiovascular: First-degree AV block, arrhythmia, postural hypotension, tachycardia, pallor, palpitations, phlebitis, ECG abnormality, ST elevation.
Nervous System: Vertigo, hypertonia, paresthesia, dizziness, somnolence.
Digestive System: Dry mouth, anorexia, tooth disorder, eructation.
Skin and Appendages: Sweating, urticaria, skin hypertrophy (nevus).
Respiratory System: Epistaxis, bronchitis, respiratory disorder.
Urogenital System: Cystitis, kidney calculus, impotence, dysmenorrhea, vaginitis, prostate disease.
Metabolic and Nutritional Disorders: Gout, edema.
Musculoskeletal System: Arthralgia, bursitis, bone pain.
Hemic and Lymphatic System: Lymphadenopathy.
Body as a Whole: Pain, unevaluable reaction, neck pain, neck rigidity, fever, chest pain, malaise.
Special Senses: Amblyopia (blurred vision), ear pain.
Angina
Cardiovascular: Palpitations, AV block, sinus bradycardia, bigeminal extrasystole, angina pectoris, hypertension, hypotension, myocardial infarct, myocardial ischemia, syncope, vasodilatation, ventricular extrasystole.
Nervous System: Abnormal thinking, neuropathy, paresthesia.
Digestive System: Diarrhea, dyspepsia, vomiting, colitis, flatulence, GI hemorrhage, stomach ulcers.
Skin and Appendages: Contact dermatitis, pruritus, sweating.
Respiratory System: Respiratory distress.
Urogenital System: Kidney failure, pyelonephritis, urinary tract infection.
Metabolic and Nutritional Disorders: Weight increase.
Musculoskeletal System: Myalgia.
Body as a Whole: Chest pain, accidental injury, infection.
Special Senses: Eye hemorrhage, ophthalmitis, otitis media, taste perversion, tinnitus.
There have been post-marketing reports of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with the use of diltiazem hydrochloride.
To report SUSPECTED ADVERSE REACTIONS, contact Alembic Pharmaceuticals Limited at 1-866-210-9797 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
OVERDOSAGE OR EXAGGERATED RESPONSE
Several literature reports have identified cases of diltiazem hydrochloride overdose, some with multiple drug ingestion, with both fatal and non-fatal outcomes. The reported events affected multiple body systems including the cardiovascular system (bradycardia, complete heart block, asystole, cardiac failure, arrhythmia, atrial fibrillation, palpitations, hypotension, ischemia, ECG changes), respiratory system (respiratory failure, hypoxia, dyspnea, pulmonary edema), central nervous system (loss of consciousness, convulsions, dizziness, confusion, agitation), gastrointestinal system (nausea, vomiting), skin and appendages (increased sweating), and other systems (hypotonia, iliac artery thrombosis, metabolic acidosis, increased blood glucose). The administration of ipecac to induce vomiting and activated charcoal to reduce drug absorption have been advocated as initial means of intervention. In addition to gastric lavage, the following measures should also be considered:
Bradycardia
Administer atropine (0.6 mg to 1 mg). If there is no response to vagal blockade, administer isoproterenol cautiously.
High-Degree AV Block
Treat as for bradycardia above. Fixed high-degree AV block should be treated with cardiac pacing.
Cardiac Failure
Administer inotropic agents (dopamine or dobutamine) and diuretics.
Hypotension
Vasopressors (e.g., dopamine or levarterenol bitartrate).
Actual treatment and dosage should depend on the severity of the clinical situation as well as the judgment and experience of the treating physician.
Due to extensive metabolism, plasma concentrations after a standard dose of diltiazem can vary over tenfold, which significantly limits their value in evaluating cases of overdosage.
Charcoal hemoperfusion has been used successfully as an adjunct therapy to hasten drug elimination. Overdoses with as much as 10.8 grams of oral diltiazem have been successfully treated using appropriate supportive care.
-
DOSAGE AND ADMINISTRATION
Hypertensive or anginal patients who are treated with other formulations of diltiazem can safely be switched to diltiazem hydrochloride extended-release capsules at the nearest equivalent total daily dose. Subsequent titration to higher or lower doses may, however, be necessary and should be initiated as clinically indicated.
Studies have shown a slight increase in the rate of absorption of diltiazem hydrochloride extended-release capsules when ingested with a high-fat breakfast; therefore, administration in the morning on an empty stomach is recommended.
Patients should be cautioned that the diltiazem hydrochloride extended-release capsules should not be opened, chewed or crushed, and should be swallowed whole.
Dosage
Hypertension
Dosages must be adjusted to each patient’s needs, starting with 180 mg or 240 mg once daily. Based on the antihypertensive effect, the dose may be adjusted as needed. Individual patients, particularly ≥60 years of age, may respond to a lower dose of 120 mg. The usual dosage range studied in clinical trials was 180 mg to 480 mg once daily.
Current clinical experience with the 540 mg dose is limited; the dose may be increased to 540 mg with little or no increased risk of adverse reactions. Doses should not exceed 540 mg once daily.
While a dose of diltiazem hydrochloride extended-release capsules given once daily may produce an antihypertensive effect similar to the same total daily dose given in divided doses, individual dose adjustment may be needed.
Dosage
Angina
Dosages for the treatment of angina should be adjusted to each patient’s needs, starting with a dose of 120 mg once daily, which may be titrated to doses of up to 480 mg once daily. When necessary, titration may be carried out over a 7 to 14 day period.
Concomitant Use with Other Cardiovascular Agents
Sublingual Nitroglycerin may be taken as required to abort acute anginal attacks during diltiazem hydrochloride therapy.
Prophylactic Nitrate Therapy – Diltiazem hydrochloride may be safely co-administered with short- and long-acting nitrates.
Beta-blockers. (See WARNINGS and PRECAUTIONS.)
Antihypertensives – Diltiazem hydrochloride has an additive antihypertensive effect when used with other antihypertensive agents. Therefore, the dosage of diltiazem hydrochloride or the concomitant antihypertensives may need to be adjusted when adding one to the other.
-
HOW SUPPLIED
Diltiazem hydrochloride extended-release capsules USP, 120 mg are pink opaque cap/light pink opaque body, hard gelatin capsules having imprinting “A250” in black ink on the cap. The capsule is filled with one white to off white oval shaped tablet.
Bottle of 100 capsules with child-resistant closure, NDC 62332-815-31
Bottle of 500 capsules, NDC 62332-815-71
Bottle of 1000 capsules, NDC 62332-815-91
Diltiazem hydrochloride extended-release capsules USP, 180 mg are lavender opaque cap/pink opaque body, hard gelatin capsules having imprinting “A251” in black ink on the cap. The capsule is filled with one white to off white oval shaped tablet.
Bottle of 100 capsules with child-resistant closure, NDC 62332-816-31
Bottle of 500 capsules, NDC 62332-816-71
Bottle of 1000 capsules, NDC 62332-816-91
Diltiazem hydrochloride extended-release capsules USP, 240 mg are blue opaque cap/pink opaque body, hard gelatin capsules having imprinting “A252” in black ink on the cap. The capsule is filled with one white to off white oval shaped tablet.
Bottle of 100 capsules with child-resistant closure, NDC 62332-817-31
Bottle of 500 capsules, NDC 62332-817-71
Bottle of 1000 capsules, NDC 62332-817-91
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Dispense in a tight, light-resistant container [see USP]. Keep tightly closed.
Keep out of the reach of children.
Rx Only
Manufactured by:
Alembic Pharmaceuticals Limited
(Formulation Division),
Panelav 389350, Gujarat, India.
Manufactured for:
Alembic Pharmaceuticals, Inc.
Bedminster, NJ 07921, USA
Revised: 09/2023 - PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 120 mg
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 180 mg
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 240 mg
-
INGREDIENTS AND APPEARANCE
DILTIAZEM HYDROCHLORIDE
diltiazem hydrochloride capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62332-815 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DILTIAZEM HYDROCHLORIDE (UNII: OLH94387TE) (DILTIAZEM - UNII:EE92BBP03H) DILTIAZEM HYDROCHLORIDE 120 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) FD&C RED NO. 40 (UNII: WZB9127XOA) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN (UNII: 2G86QN327L) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) SHELLAC (UNII: MB5IUD6JUA) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color PINK (Pink opaque cap) , PINK (Light pink opaque body) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code A250 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62332-815-31 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/28/2024 2 NDC:62332-815-71 500 in 1 BOTTLE; Type 0: Not a Combination Product 10/28/2024 3 NDC:62332-815-91 1000 in 1 BOTTLE; Type 0: Not a Combination Product 10/28/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218587 10/28/2024 DILTIAZEM HYDROCHLORIDE
diltiazem hydrochloride capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62332-816 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DILTIAZEM HYDROCHLORIDE (UNII: OLH94387TE) (DILTIAZEM - UNII:EE92BBP03H) DILTIAZEM HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) FD&C RED NO. 40 (UNII: WZB9127XOA) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN (UNII: 2G86QN327L) SHELLAC (UNII: MB5IUD6JUA) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Product Characteristics Color PURPLE (Lavender opaque cap) , PINK (Pink opaque body) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code A251 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62332-816-31 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/28/2024 2 NDC:62332-816-71 500 in 1 BOTTLE; Type 0: Not a Combination Product 10/28/2024 3 NDC:62332-816-91 1000 in 1 BOTTLE; Type 0: Not a Combination Product 10/28/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218587 10/28/2024 DILTIAZEM HYDROCHLORIDE
diltiazem hydrochloride capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62332-817 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DILTIAZEM HYDROCHLORIDE (UNII: OLH94387TE) (DILTIAZEM - UNII:EE92BBP03H) DILTIAZEM HYDROCHLORIDE 240 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) FD&C RED NO. 40 (UNII: WZB9127XOA) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN (UNII: 2G86QN327L) SHELLAC (UNII: MB5IUD6JUA) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Product Characteristics Color BLUE (Blue opaque cap) , PINK (Pink opaque body) Score no score Shape CAPSULE Size 23mm Flavor Imprint Code A252 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62332-817-31 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/28/2024 2 NDC:62332-817-71 500 in 1 BOTTLE; Type 0: Not a Combination Product 10/28/2024 3 NDC:62332-817-91 1000 in 1 BOTTLE; Type 0: Not a Combination Product 10/28/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218587 10/28/2024 Labeler - Alembic Pharmaceuticals Inc. (079288842) Establishment Name Address ID/FEI Business Operations Alembic Pharmaceuticals Limited 650574671 MANUFACTURE(62332-815, 62332-816, 62332-817)