Label: ALBADRY PLUS- novobiocin sodium and penicillin g procaine injection, solution
- NDC Code(s): 54771-3139-1, 54771-3139-2, 54771-3139-9
- Packager: Zoetis Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated June 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- VETERINARY INDICATIONS
- CAUTION
- DESCRIPTION
- INDICATIONS FOR USE
- WARNINGS
- PRECAUTION
- DOSAGE
-
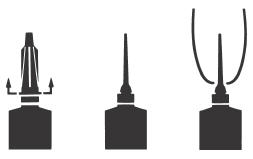
DIRECTIONS FOR USING THE FLEXI-TUBE® SYSTEM
The FLEXI-TUBE is designed to provide the choice of either insertion of the full cannula, as has traditionally been practiced, or insertion of no more than ⅛ inch of the cannula, as recommended by the National Mastitis Council.
-
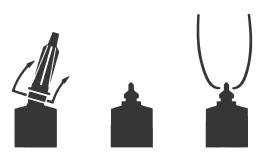
Full Insertion: Remove the blue end cap by pulling straight up as shown. Gently insert the full cannula into the teat canal; carefully infuse the product.
-
-
Partial Insertion: Remove both the blue end cap and the red cannula by pushing sideways as shown. Gently insert the exposed blue tip into the teat canal; carefully infuse the product.
-
-
Full Insertion: Remove the blue end cap by pulling straight up as shown. Gently insert the full cannula into the teat canal; carefully infuse the product.
-
ADMINISTRATION
At the time of drying off, but not less than 30 days prior to calving, milk the udder dry. Wash the teats and udder thoroughly with warm water containing a suitable dairy antiseptic. Dry the teats and udder thoroughly. Infuse each quarter using the following procedure. Using the alcohol pads provided, scrub each teat end clean using a separate pad for each teat. Warm ALBADRY PLUS Suspension to body temperature and shake thoroughly. Choose the desired insertion length (full or partial) and insert tip into teat canal. Instill entire contents into the quarter. Massage the udder after treatment to distribute the ALBADRY PLUS Suspension throughout the quarters. Using a suitable teat dip, dip all teats following treatment.
- SPL UNCLASSIFIED SECTION
- STORAGE CONDITIONS
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 10 mL Syringe Label
-
INGREDIENTS AND APPEARANCE
ALBADRY PLUS
novobiocin sodium and penicillin g procaine injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-3139 Route of Administration INTRAMAMMARY Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NOVOBIOCIN SODIUM (UNII: Q9S9NQ5YIY) (NOVOBIOCIN - UNII:17EC19951N) NOVOBIOCIN 400 mg in 10 mL PENICILLIN G PROCAINE (UNII: 17R794ESYN) (PENICILLIN G - UNII:Q42T66VG0C) PENICILLIN G 200000 [iU] in 10 mL Inactive Ingredients Ingredient Name Strength CHLOROBUTANOL (UNII: HM4YQM8WRC) 50 mg in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-3139-1 12 in 1 CARTON 1 NDC:54771-3139-9 10 mL in 1 SYRINGE 2 NDC:54771-3139-2 144 in 1 PAIL 2 NDC:54771-3139-9 10 mL in 1 SYRINGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA055098 01/07/1983 Labeler - Zoetis Inc. (828851555)