Label: FEXOFENADINE HYDROCHLORIDE tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 62756-542-64, 62756-542-66, 62756-542-74, 62756-542-76, view more62756-542-77, 62756-542-78, 62756-542-83, 62756-542-88, 62756-543-64, 62756-543-66, 62756-543-74, 62756-543-76, 62756-543-77, 62756-543-78, 62756-543-83, 62756-543-88, 62756-545-64, 62756-545-66, 62756-545-74, 62756-545-76, 62756-545-77, 62756-545-78, 62756-545-83, 62756-545-88 - Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 26, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
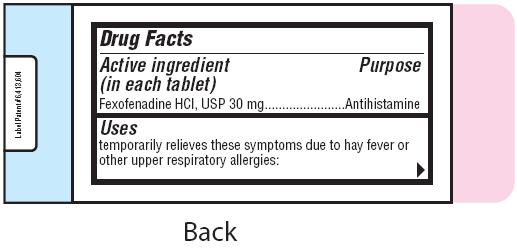
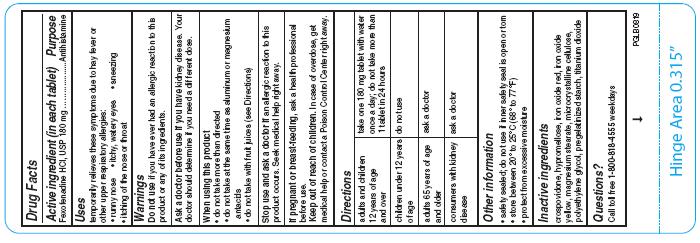
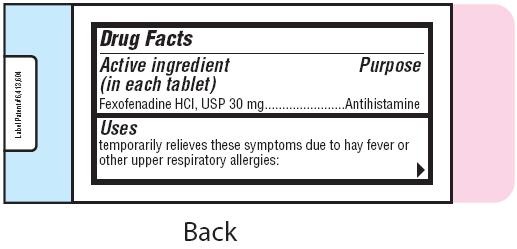
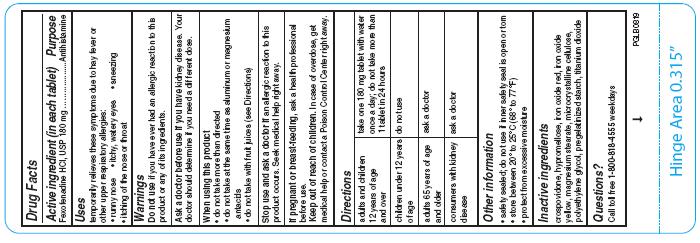
- Active ingredient (in each tablet)
- Purpose
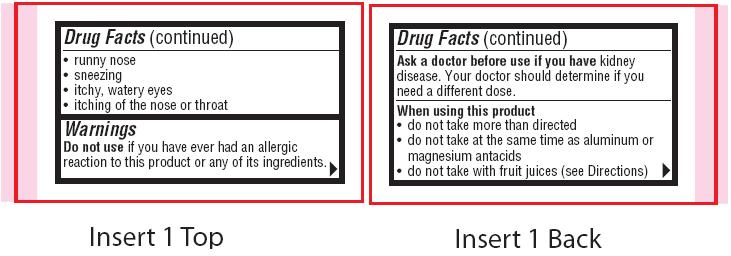
- Uses
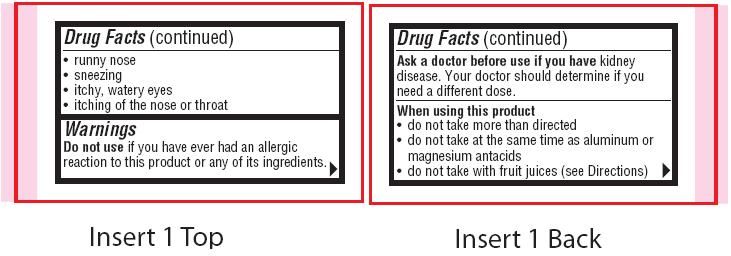
- Warnings
- Ask a doctor before use if you have
- When using this product
- Stop use and ask doctor if
- If pregnant or breast-feeding
- Keep out of reach of children.
-
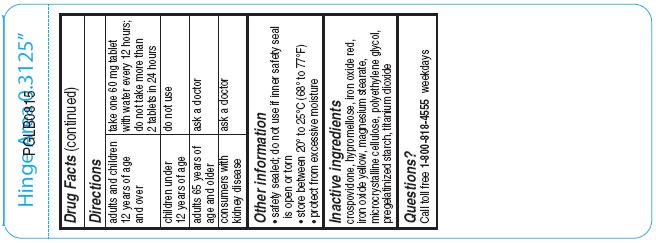
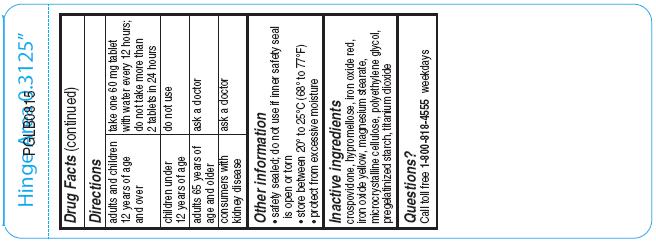
Directions
For 30mg:
adults and children 12 years of age and over
take two 30 mg tablets with water every 12 hours; do not take more than 4 tablets in 24 hours
children 6 to under 12 years of age take one 30 mg tablet with water every 12 hours; do not take more than 2 tablets in 24 hours
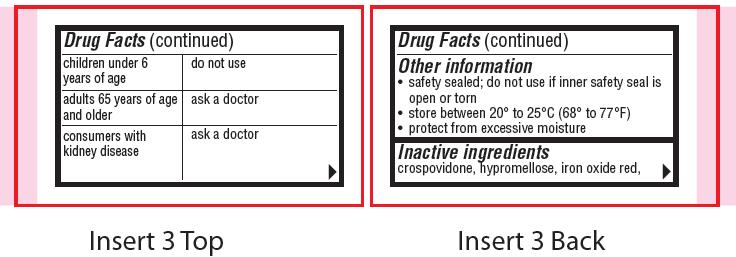
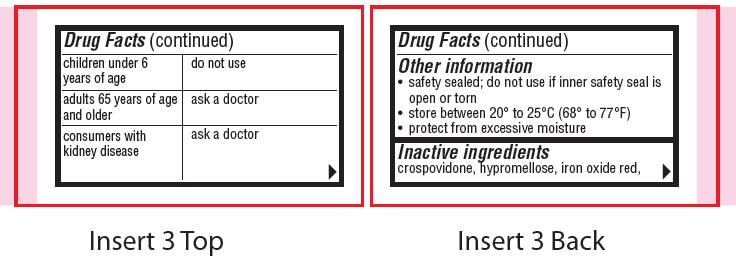
children under 6 years of age
do not use
adults 65 years of age and older
ask a doctor
consumers with kidney disease
ask a doctor
For 60mg:
For 180mg:
adults and children 12 years of age and over
take one 180 mg tablet with water once a day; do not take more than 1 tablet in 24 hours
children under 12 years of age
do not use
adults 65 years of age and older
ask a doctor
consumers with kidney disease
ask a doctor
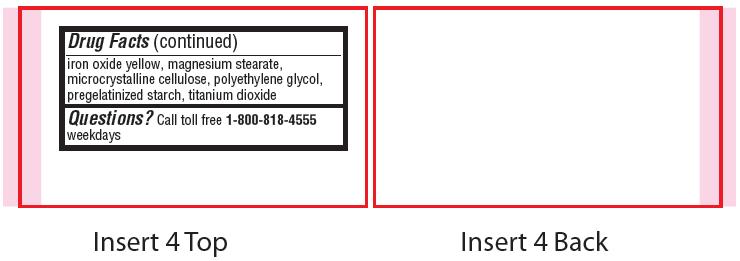
- Other information
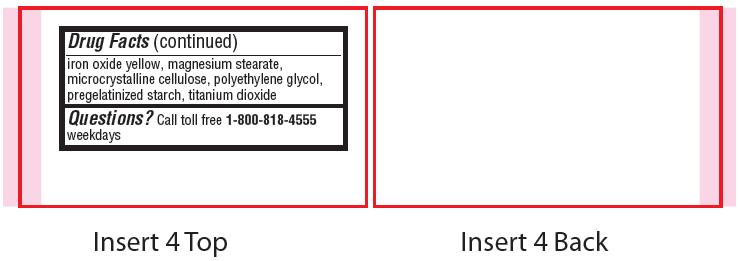
- Inactive ingredients
- Questions?
-
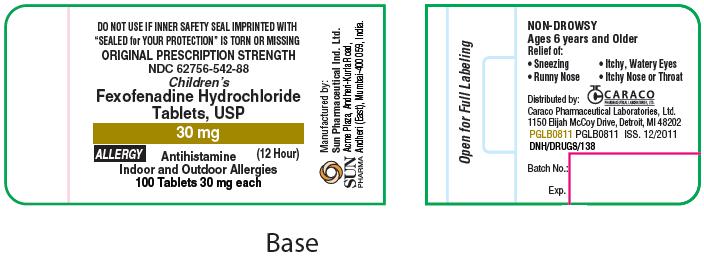
Principal Display Panel
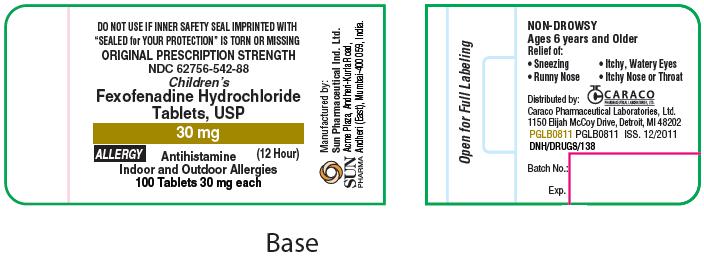
For 30 mg Allergy:
ORIGINAL PRESCRIPTION STRENGTH
NDC 62756-542-88
Children's
Fexofenadine Hydrochloride Tablets, USP
30 mg
ALLERGY
(12 Hour)
Antihistamine
Indoor and Outdoor Allergies
100 Tablets 30 mg each
SUN PHARMA

For 60 mg Allergy:
NDC 62756-543-88
Fexofenadine Hydrochloride Tablets, USP
60 mg
ALLERGY
(12 Hour)
Antihistamine
Indoor and Outdoor Allergies
100 Tablets 60 mg each
SUN PHARMA


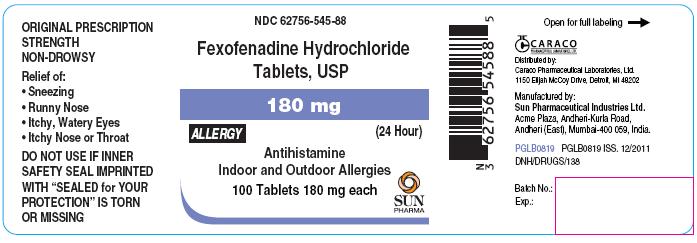
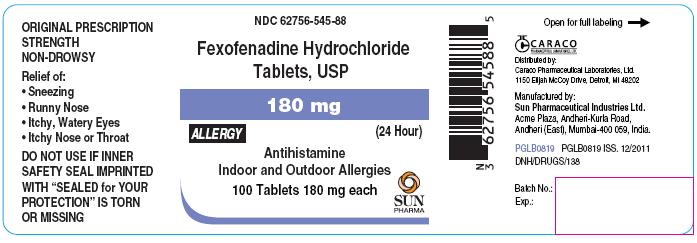
For 180 mg Allergy:
NDC 62756-545-88
Fexofenadine Hydrochloride Tablets, USP
180 mg
ALLERGY
(24 Hour)
Antihistamine
Indoor and Outdoor Allergies
100 Tablets 180 mg each
SUN PHARMA
-
Principal Display Panel
For 30 mg Allergy:
NDC 62756-542-74
ORIGINAL PRESCRIPTION STRENGTH
NON-DROWSY
Children's
Fexofenadine Hydrochloride Tablets, USP
30 mg
ALLERGY
(12 Hour)
Antihistamine
Indoor and Outdoor Allergies
Ages 6 years and Older
Relief of:
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Nose or Throat
5 (1 x 5)Tablets 30 mg each
SUN PHARMA
DO NOT USE IF INDIVIDUAL BLISTER UNIT IS OPEN OR TORN

-
Principal Display Panel
For 60 mg Allergy:
NDC 62756-543-74
ORIGINAL PRESCRIPTION STRENGTH
NON-DROWSY
Fexofenadine Hydrochloride Tablets, USP
60 mg
ALLERGY
(12 Hour)
Antihistamine
Indoor and Outdoor Allergies
Relief of:
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Nose or Throat
5 (1 x 5)Tablets 60 mg each
SUN PHARMA
DO NOT USE IF INDIVIDUAL BLISTER UNIT IS OPEN OR TORN

-
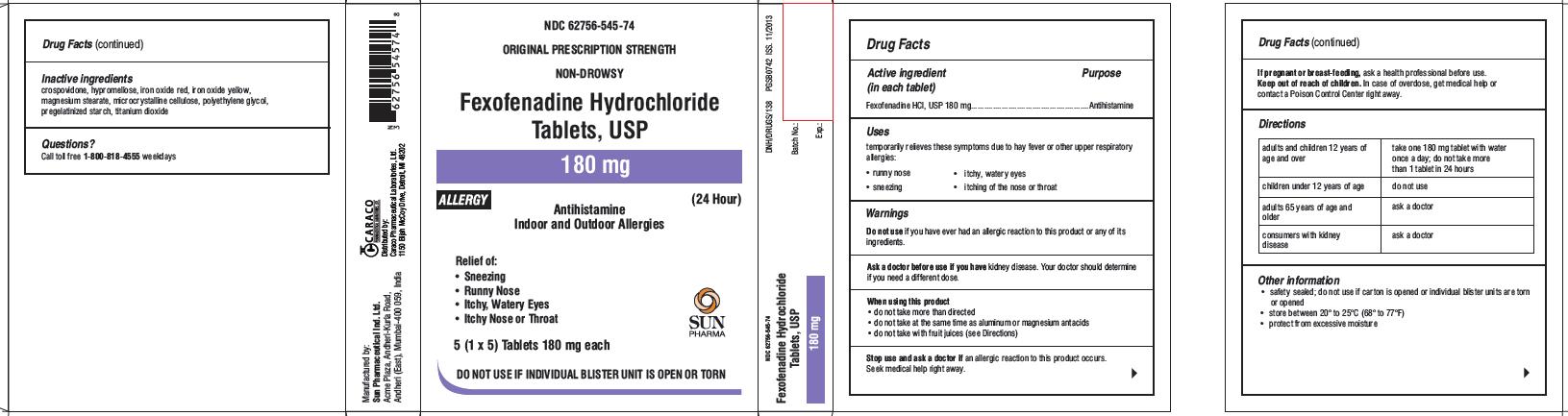
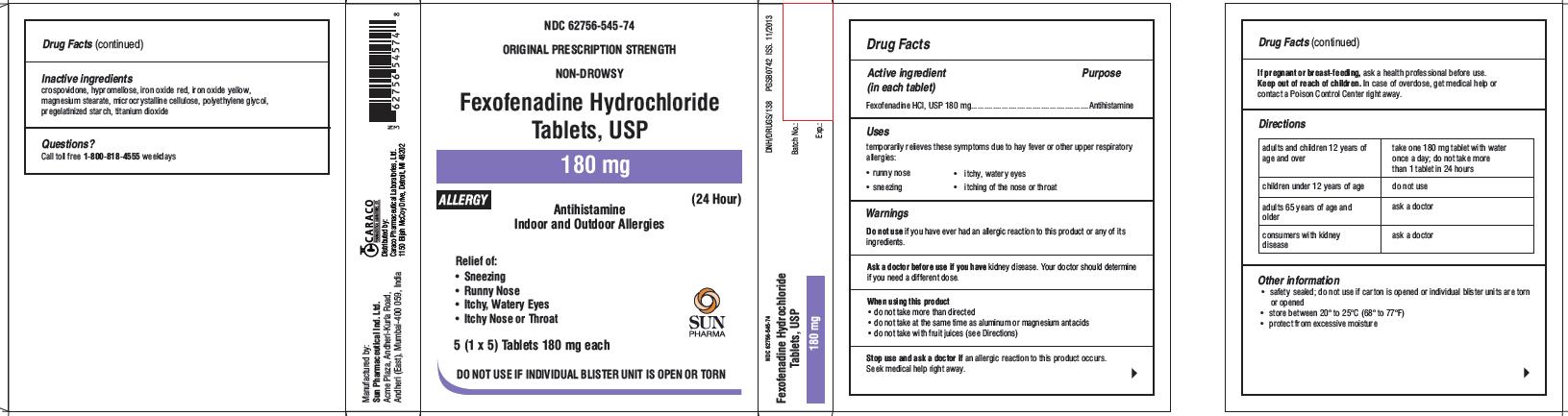
Principal Display Panel
For 180 mg Allergy:
NDC 62756-545-74
ORIGINAL PRESCRIPTION STRENGTH
NON-DROWSY
Fexofenadine Hydrochloride Tablets, USP
180 mg
ALLERGY
(24 Hour)
Antihistamine
Indoor and Outdoor Allergies
Relief of:
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Nose or Throat
5 (1 x 5)Tablets 180 mg each
SUN PHARMA
DO NOT USE IF INDIVIDUAL BLISTER UNIT IS OPEN OR TORN
-
INGREDIENTS AND APPEARANCE
FEXOFENADINE HYDROCHLORIDE
fexofenadine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62756-542 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 30 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) CROSPOVIDONE (UNII: 68401960MK) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color PINK Score no score Shape ROUND (circular) Size 6mm Flavor Imprint Code 542 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62756-542-83 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/06/2012 2 NDC:62756-542-88 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/06/2012 3 NDC:62756-542-74 1 in 1 CARTON 02/06/2012 3 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:62756-542-66 2 in 1 CARTON 02/06/2012 4 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 5 NDC:62756-542-77 9 in 1 CARTON 02/06/2012 5 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 6 NDC:62756-542-64 5 in 1 CARTON 02/06/2012 6 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 7 NDC:62756-542-76 10 in 1 CARTON 02/06/2012 7 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 8 NDC:62756-542-78 15 in 1 CARTON 02/06/2012 8 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091567 02/06/2012 FEXOFENADINE HYDROCHLORIDE
fexofenadine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62756-543 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 60 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) CROSPOVIDONE (UNII: 68401960MK) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color PINK Score no score Shape OVAL Size 12mm Flavor Imprint Code 543 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62756-543-83 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/06/2012 2 NDC:62756-543-88 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/06/2012 3 NDC:62756-543-74 1 in 1 CARTON 02/06/2012 3 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:62756-543-66 2 in 1 CARTON 02/06/2012 4 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 5 NDC:62756-543-77 9 in 1 CARTON 02/06/2012 5 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 6 NDC:62756-543-64 5 in 1 CARTON 02/06/2012 6 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 7 NDC:62756-543-76 10 in 1 CARTON 02/06/2012 7 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 8 NDC:62756-543-78 15 in 1 CARTON 02/06/2012 8 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091567 02/06/2012 FEXOFENADINE HYDROCHLORIDE
fexofenadine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62756-545 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) CROSPOVIDONE (UNII: 68401960MK) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color PINK Score no score Shape CAPSULE Size 17mm Flavor Imprint Code 545 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62756-545-83 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/06/2012 2 NDC:62756-545-88 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/06/2012 3 NDC:62756-545-74 1 in 1 CARTON 02/06/2012 3 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:62756-545-66 2 in 1 CARTON 02/06/2012 4 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 5 NDC:62756-545-77 9 in 1 CARTON 02/06/2012 5 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 6 NDC:62756-545-64 5 in 1 CARTON 02/06/2012 6 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 7 NDC:62756-545-76 10 in 1 CARTON 02/06/2012 7 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 8 NDC:62756-545-78 15 in 1 CARTON 02/06/2012 8 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091567 02/06/2012 Labeler - Sun Pharmaceutical Industries, Inc. (146974886) Establishment Name Address ID/FEI Business Operations Sun Pharmaceutical Industries Limited 650445203 ANALYSIS(62756-542, 62756-543, 62756-545) , LABEL(62756-542, 62756-545) , MANUFACTURE(62756-542, 62756-543, 62756-545) , PACK(62756-542, 62756-545)