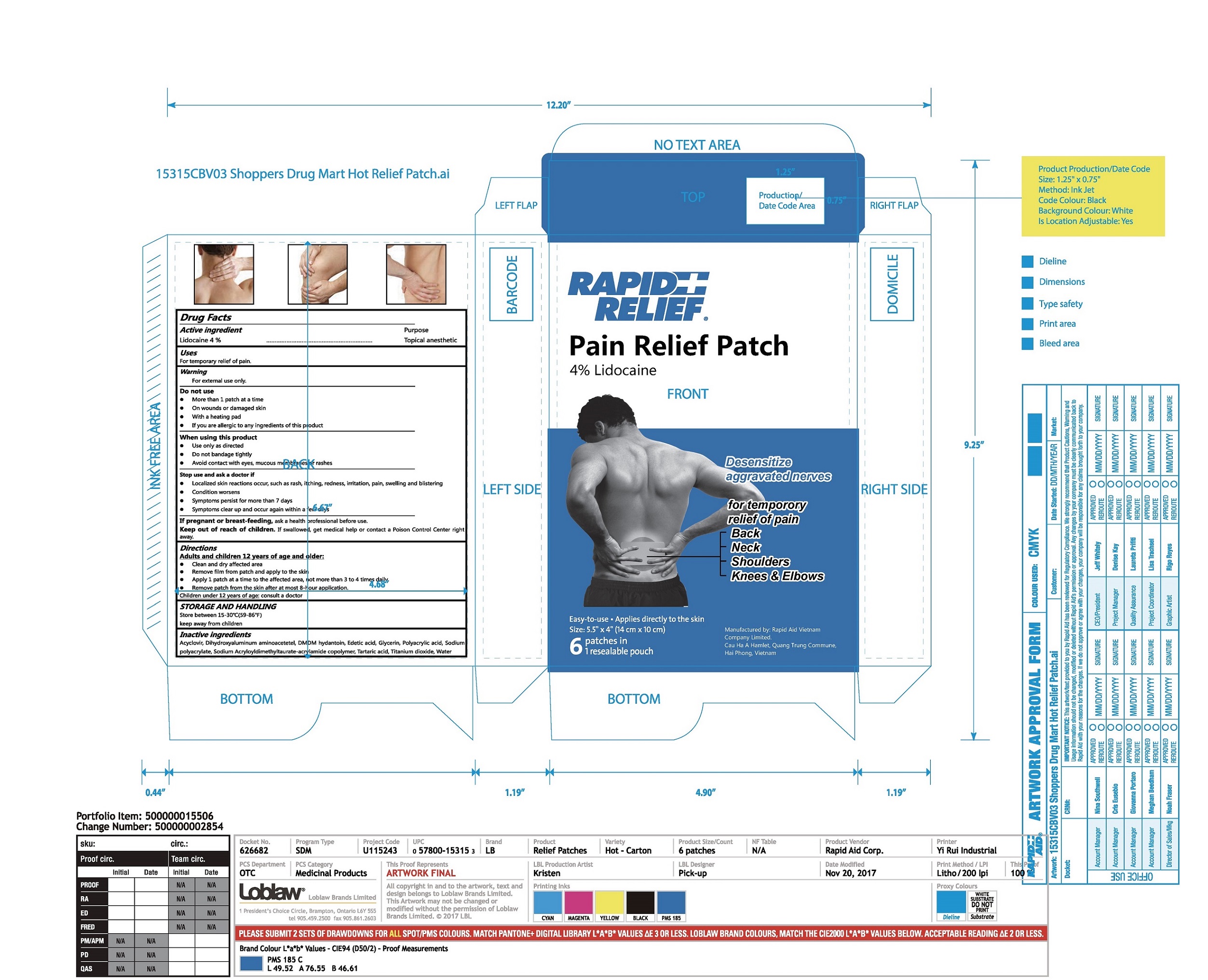
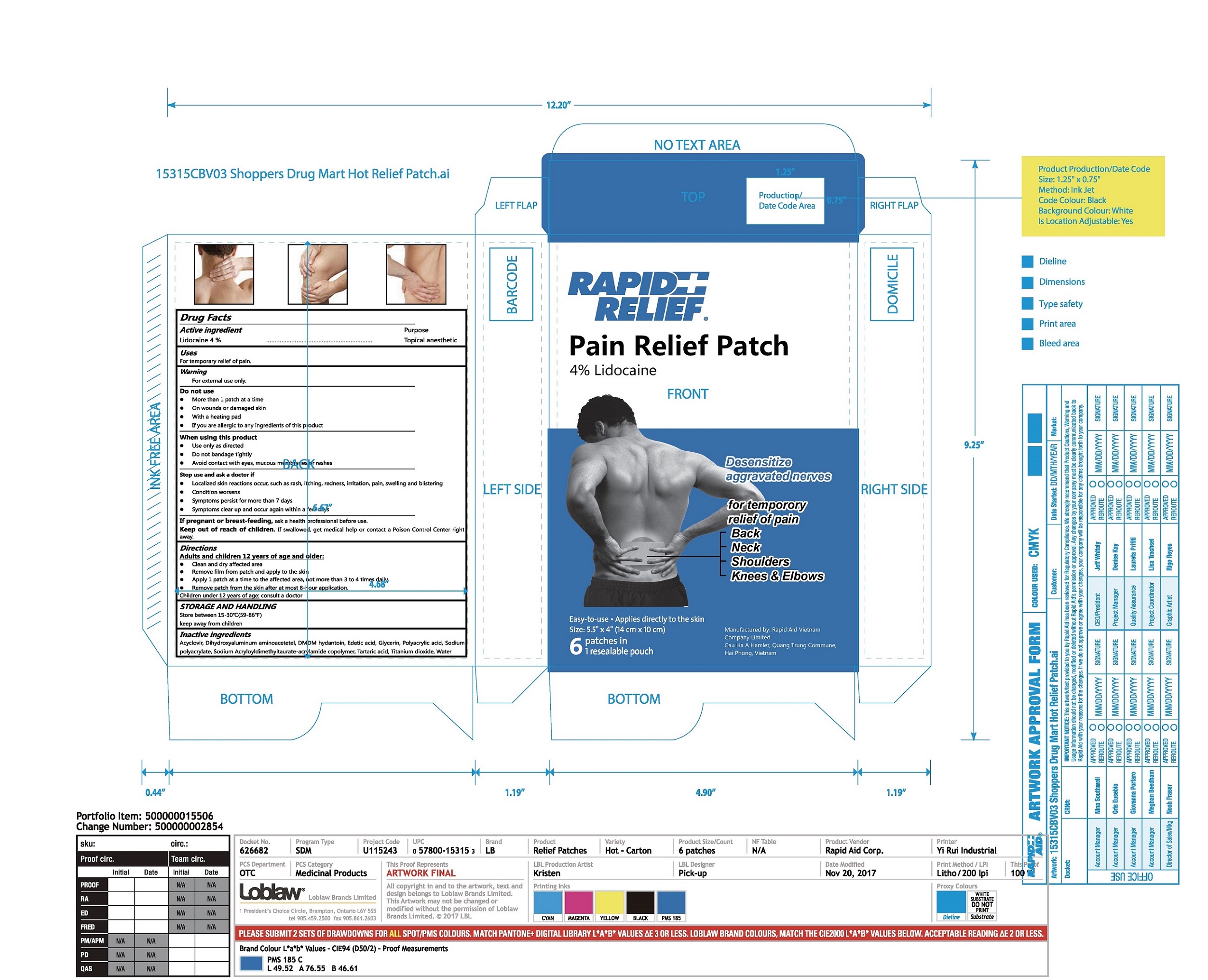
Label: RAPID RELIEF PAIN RELIEF PATCH- lidocaine patch
- NDC Code(s): 83569-002-01
- Packager: RAPID AID VIET NAM CO., LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
- When using this product:
- Stop use and ask a doctor if:
- KEEP OUT OF REACH OF CHILDREN
-
Directions
Adults and children 12 years of age and older:
Clean and dry affected area
Remove film from patch and apply to the skin
Apply 1 patch at a time to the affected area, not more than 3 to 4 times daily.
Remove patch from the skin after at most 8-hour application.
Children under 12 years of age: consult a doctor - STORAGE AND HANDLING
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
RAPID RELIEF PAIN RELIEF PATCH
lidocaine patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83569-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Lidocaine (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) Lidocaine 4 g in 100 g Inactive Ingredients Ingredient Name Strength Acyclovir (UNII: X4HES1O11F) POLYACRYLIC ACID (450000 MW) (UNII: KD3S7H73D3) Dihydroxyaluminum aminoacetate (UNII: DO250MG0W6) Glycerin (UNII: PDC6A3C0OX) Water (UNII: 059QF0KO0R) DMDM hydantoin (UNII: BYR0546TOW) Edetic acid (UNII: 9G34HU7RV0) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) SODIUM ACRYLOYLDIMETHYLTAURATE-ACRYLAMIDE COPOLYMER (1:1; 90000-150000 MPA.S) (UNII: 5F4963KLHS) Tartaric acid (UNII: W4888I119H) Titanium dioxide (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83569-002-01 1 in 1 BOX 03/06/2024 1 6 in 1 POUCH 1 4 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/06/2024 Labeler - RAPID AID VIET NAM CO., LTD (673067008) Registrant - RAPID AID VIET NAM CO., LTD (673067008) Establishment Name Address ID/FEI Business Operations RAPID AID VIET NAM CO., LTD 673067008 manufacture(83569-002)