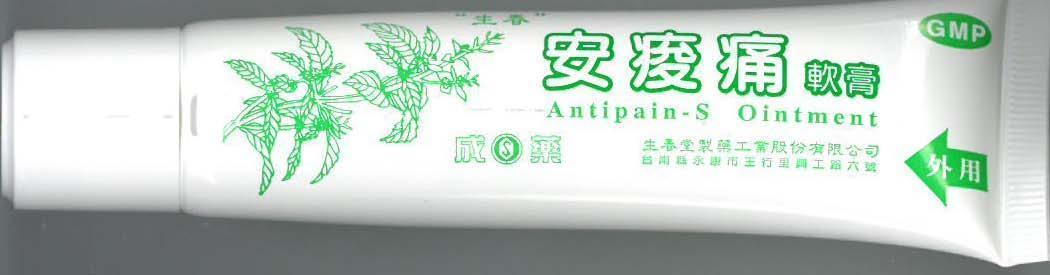
Label: ANTIPAIN-S- radix clematidis, semen momordicae, myrrha, olibanum, menthol, methyl salicylate ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 69165-201-21, 69165-201-51 - Packager: GEORGE L. RODRIGUEZ M.D., PC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated August 12, 2014
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- KEEP OUT OF REACH OF CHILDREN
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTIPAIN-S
radix clematidis, semen momordicae, myrrha, olibanum, menthol, methyl salicylate ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69165-201 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLEMATIS HEXAPETALA WHOLE (UNII: 8F900R77RS) (CLEMATIS HEXAPETALA WHOLE - UNII:8F900R77RS) CLEMATIS HEXAPETALA WHOLE 30 mg in 1 g MOMORDICA CHARANTIA WHOLE (UNII: 83M15WOS0F) (MOMORDICA CHARANTIA WHOLE - UNII:83M15WOS0F) MOMORDICA CHARANTIA WHOLE 15 mg in 1 g MYRRH (UNII: JC71GJ1F3L) (MYRRH - UNII:JC71GJ1F3L) MYRRH 20 mg in 1 g FRANKINCENSE OIL (UNII: 67ZYA5T02K) (FRANKINCENSE OIL - UNII:67ZYA5T02K) FRANKINCENSE OIL 20 mg in 1 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 50 mg in 1 g Inactive Ingredients Ingredient Name Strength YELLOW WAX (UNII: 2ZA36H0S2V) RICINUS COMMUNIS WHOLE (UNII: Q94QWM85RF) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69165-201-51 1 in 1 BOX 1 NDC:69165-201-21 35 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/12/2014 Labeler - GEORGE L. RODRIGUEZ M.D., PC (028675061)