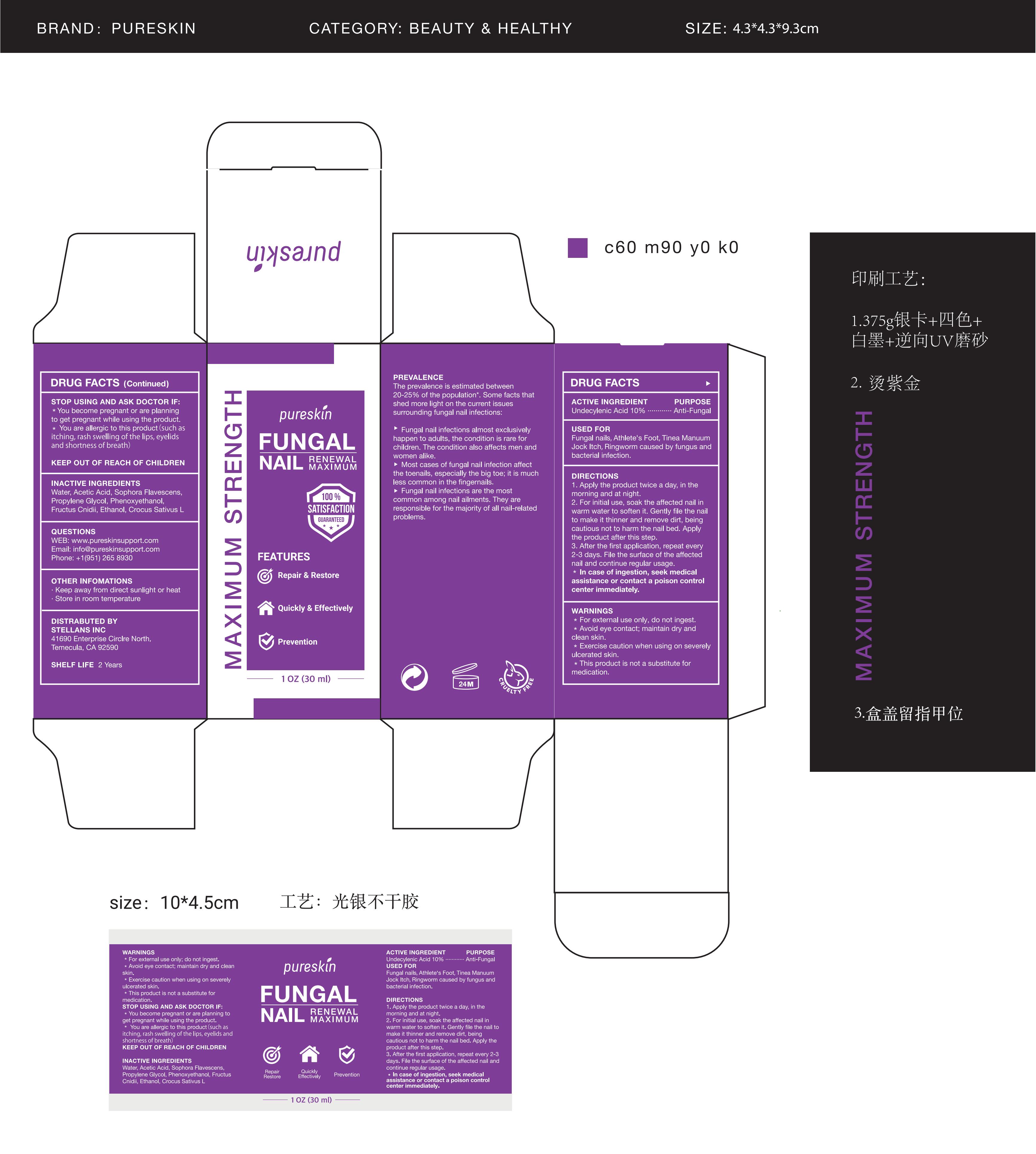
Label: PURESKIN FUNGAL NAIL RENEWAL- fungal nail renewal liquid
- NDC Code(s): 83565-003-01
- Packager: Stellans Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Ask Doctor
- Keep Oot Of Reach Of Children
-
Directions
1. Apply the product twice a day, in the morning and at night.
2. For initial use, soak the affected nail in warm water to soften it. Gently file the nail to make it thinner and remove dirt, being cautious not to harm the nail bed. Apply the product after this step.
3. After the first application, repeat every 2-3 days. File the surface of the affected nail and continue regular usage.
In case of ingestion, seek medical assistance or contact a poison control center immediately. - Other information
- Inactive ingredients
- Questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PURESKIN FUNGAL NAIL RENEWAL
fungal nail renewal liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83565-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UNDECYLENIC ACID (UNII: K3D86KJ24N) (UNDECYLENIC ACID - UNII:K3D86KJ24N) UNDECYLENIC ACID 10 g in 100 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) SOPHORA FLAVESCENS WHOLE (UNII: X8KX602M5L) PHENOXYETHANOL (UNII: HIE492ZZ3T) CROCUS SATIVUS WHOLE (UNII: Z5C927G4XF) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CNIDIUM MONNIERI FRUIT (UNII: V1IA3S3CUS) ACETIC ACID (UNII: Q40Q9N063P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83565-003-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/30/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M005 08/30/2023 Labeler - Stellans Inc. (111157321) Establishment Name Address ID/FEI Business Operations Stellans Inc. 111157321 label(83565-003) , manufacture(83565-003)